From VALID Act Definitions section
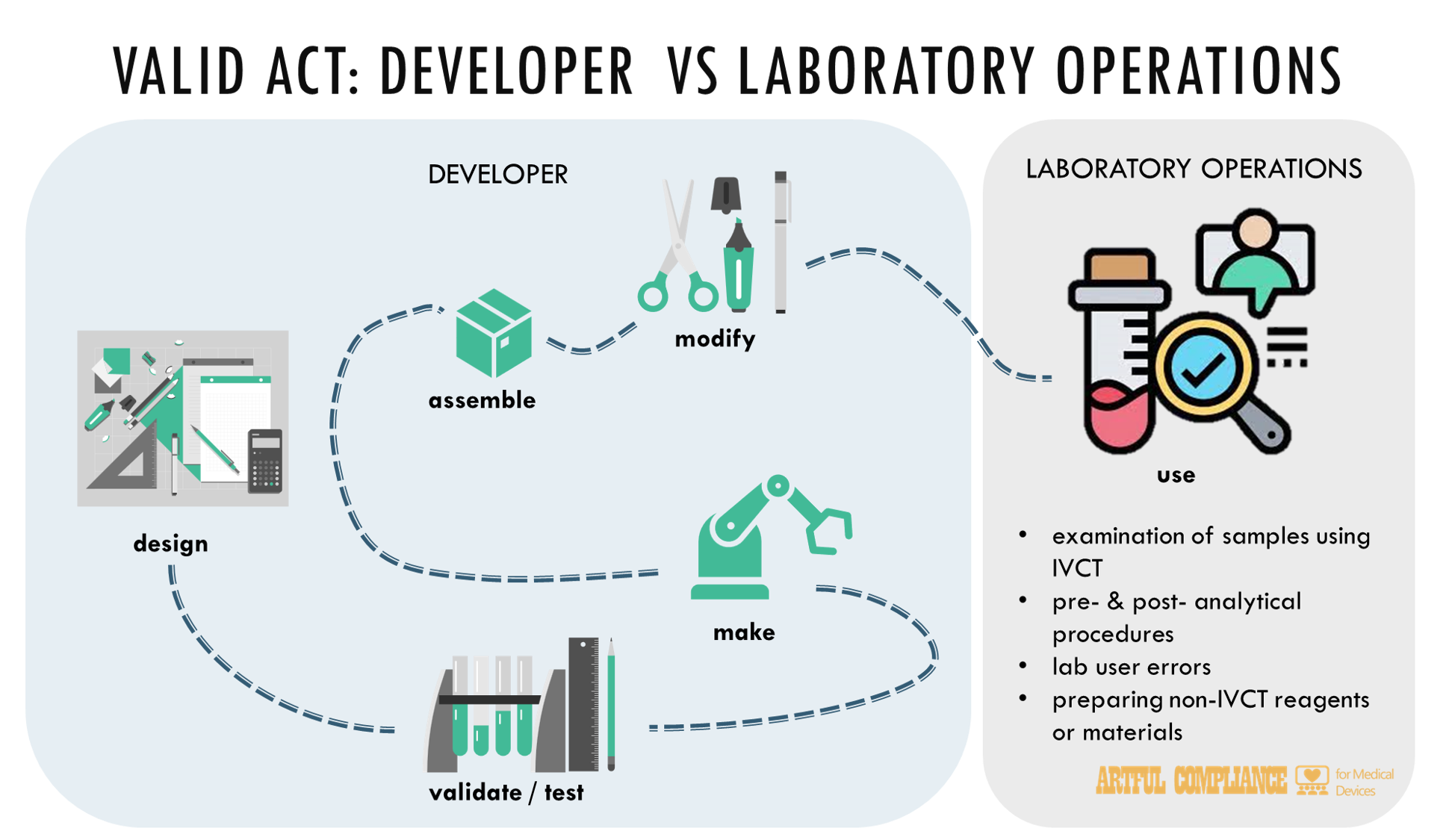
“(6) DEVELOP.—The term ‘develop’, with respect to an in vitro clinical test, means—
“(A) designing, validating, producing, manufacturing, remanufacturing, propagating, or assembling an in vitro clinical test;
“(B) importing an in vitro clinical test;
“(C) modifying an in vitro clinical test initially developed by a different person in a manner that—
“(i) changes any of the listing elements that define indications for use specified in paragraph (10)*, performance claims, or, as applicable, the safety of such in vitro clinical test; or
“(ii) affects the analytical or clinical validity of the in vitro clinical test as intended by the developer; or
“(D) adopting, using, or disseminating for use as an in vitro clinical test an article not previously intended for clinical use.
“(7) DEVELOPER.—The term ‘developer’ means a person who engages in an activity described in paragraph (6) for clinical use.
{*Substance or substances, Test method, test purpose(s), diseases or conditions for use, context of use}
“(13) LABORATORY OPERATIONS.—The term ‘laboratory operations’—
“(A) means the conduct of a laboratory examination or other laboratory procedure on materials derived from the human body, including the conduct of an in vitro clinical test and associated activities within or under the oversight of a laboratory and not related to the design of an in vitro clinical test; and
“(B) includes—
“(i) performing pre-analytical and post-analytical processes for an in vitro clinical test;
“(ii) conducting standard operating procedures; and
“(iii) preparing reagents or other test materials that do not meet the definition of a in vitro clinical test for clinical use under section 201(ss).
Recent Comments