
00095 Cybersecurity in Medical Devices- Scope
Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions
- Scope
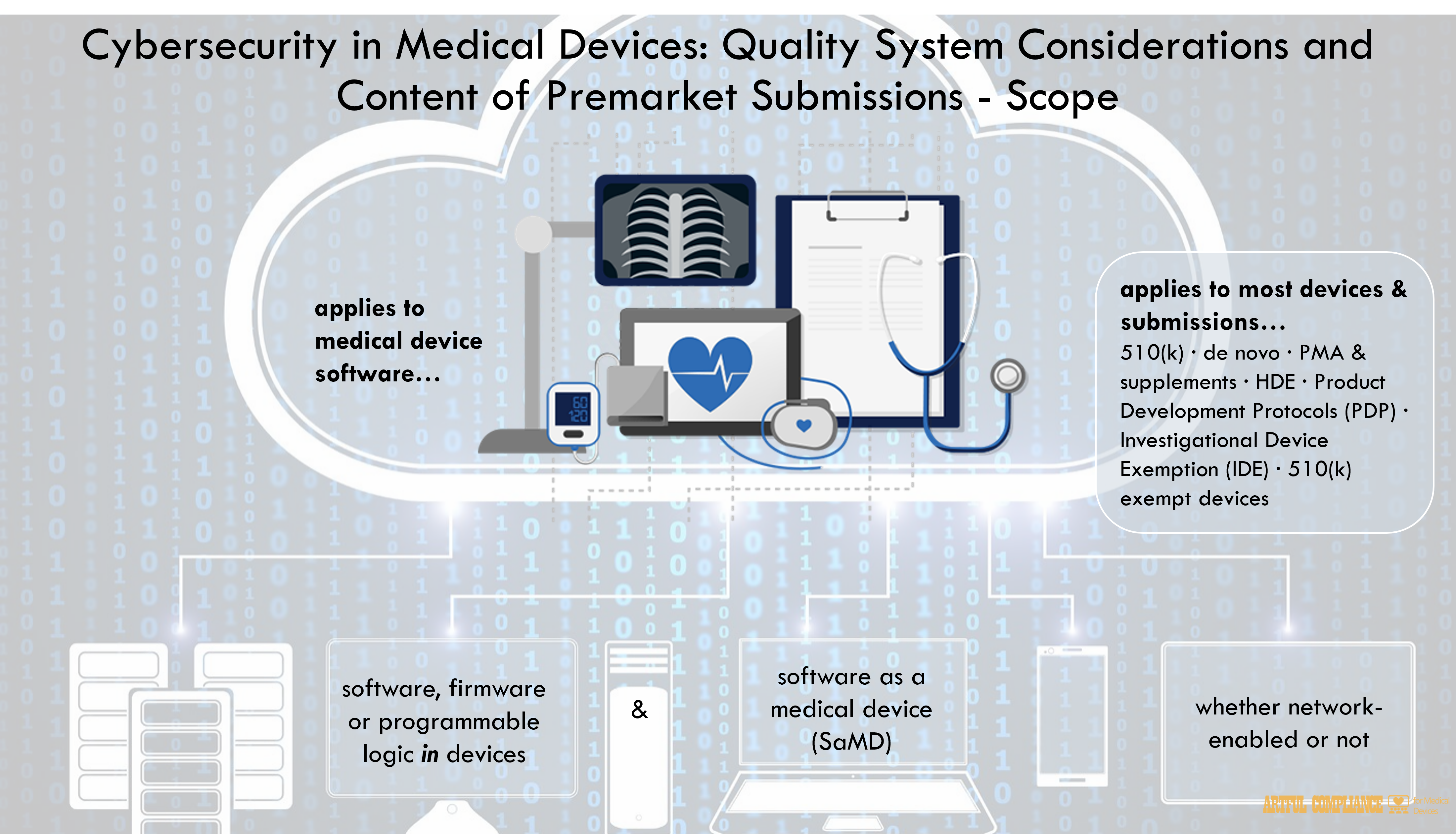
This guidance document is applicable to devices that contain software (including firmware) or programmable logic, as well as software as a medical device (SaMD). The guidance is not limited to devices that are network-enabled or contain other connected capabilities. This
guidance describes recommendations regarding the cybersecurity information to be submitted for devices under the following premarket submission types
· Premarket Notification (510(k)) submissions;
· De Novo requests;
· Premarket Approval Applications (PMAs) and PMA supplements;
· Product Development Protocols (PDPs);
· Investigational Device Exemption (IDE) submissions; and
· Humanitarian Device Exemption (HDE) submissions.This guidance applies to all types of devices within the meaning of section 201(h) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) whether or not they require a premarket submission. Therefore, the information in this guidance should also be considered for understanding FDA’s recommendations for devices for which a premarket submission is not required (e.g., for 510(k)-exempt devices).
As IDE submissions have a different benefit-risk threshold and are not marketing authorizations, specific considerations for IDE submission documentation are provided in Appendix
Appendix 4 contains terminology used throughout the guidance
Recent Comments