PPT-00093 REV01 21 CFR Part 801 Labeling
CHAPTER I–FOOD AND DRUG ADMINISTRATION
DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUBCHAPTER H – MEDICAL DEVICES
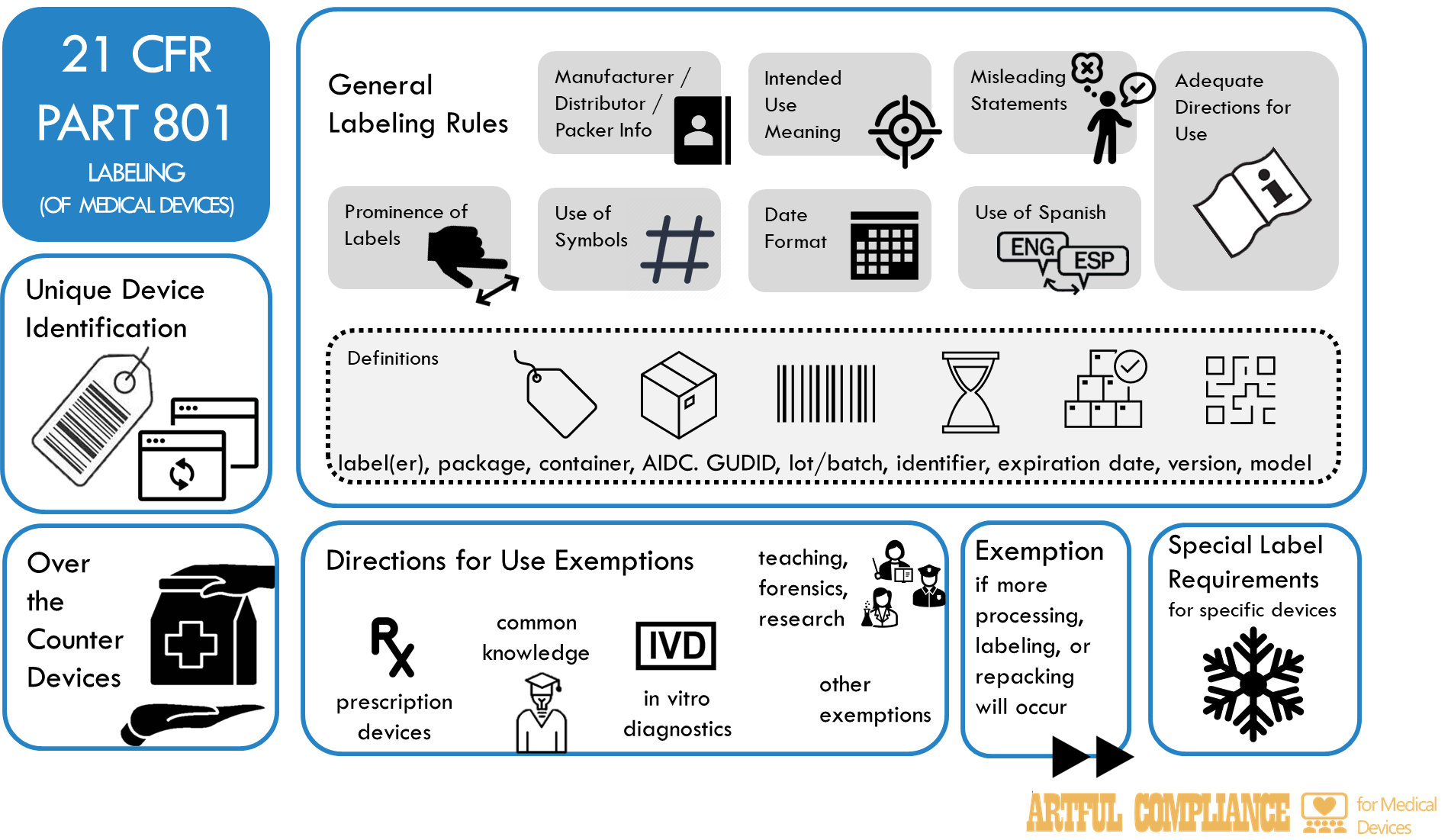
§ 801.1 – Medical devices; name and place of business of manufacturer, packer or distributor.
§ 801.3 – Definitions.
§ 801.4 – Meaning of intended uses.
§ 801.5 – Medical devices; adequate directions for use.
§ 801.6 – Medical devices; misleading statements.
§ 801.15 – Medical devices; prominence of required label statements; use of symbols in labeling.
§ 801.16 – Medical devices; Spanish-language version of certain required statements.
§ 801.18 – Format of dates provided on a medical device label.
Subpart B – Labeling Requirements for Unique Device Identification
§ 801.20 – Label to bear a unique device identifier.
§ 801.30 – General exceptions from the requirement for the label of a device to bear a unique device identifier.
§ 801.35 – Voluntary labeling of a device with a unique device identifier.
§ 801.40 – Form of a unique device identifier.
§ 801.45 – Devices that must be directly marked with a unique device identifier.
§ 801.50 – Labeling requirements for stand-alone software.
§ 801.55 – Request for an exception from or alternative to a unique device identifier requirement.
§ 801.57 – Discontinuation of legacy FDA identification numbers assigned to devices.
Subpart C – Labeling Requirements for Over-the-Counter Devices
§ 801.60 – Principal display panel.
§ 801.61 – Statement of identity.
§ 801.62 – Declaration of net quantity of contents.
§ 801.63 – Medical devices; warning statements for devices containing or manufactured with chlorofluorocarbons and other class I ozone-depleting substances.
Subpart D – Exemptions From Adequate Directions for Use
§ 801.109 – Prescription devices.
§ 801.110 – Retail exemption for prescription devices.
§ 801.116 – Medical devices having commonly known directions.
§ 801.119 – In vitro diagnostic products.
§ 801.122 – Medical devices for processing, repacking, or manufacturing.
§ 801.125 – Medical devices for use in teaching, law enforcement, research, and analysis.
§ 801.127 – Medical devices; expiration of exemptions.
§ 801.128 – Exceptions or alternatives to labeling requirements for medical devices held by the Strategic National Stockpile.
Subpart E – Other Exemptions
§ 801.150 – Medical devices; processing, labeling, or repacking.
Subparts F-G [Reserved]
Subpart H – Special Requirements for Specific Devices
§ 801.405 – Labeling of articles intended for lay use in the repairing and/or refitting of dentures.
§ 801.410 – Use of impact-resistant lenses in eyeglasses and sunglasses.
§ 801.415 – Maximum acceptable level of ozone.
§ 801.417 – Chlorofluorocarbon propellants.
§ 801.422 – Prescription hearing aid labeling.
§ 801.430 – User labeling for menstrual tampons.
§ 801.433 – Warning statements for prescription and restricted device products containing or manufactured with chlorofluorocarbons or other ozone-depleting substances.
§ 801.435 – User labeling for latex condoms.
§ 801.437 – User labeling for devices that contain natural rubber.

Recent Comments