00087 VALID Act Special Pre-Market Review
From Valid Act “SEC. 587B. Premarket review.
“(d) Special premarket review.—
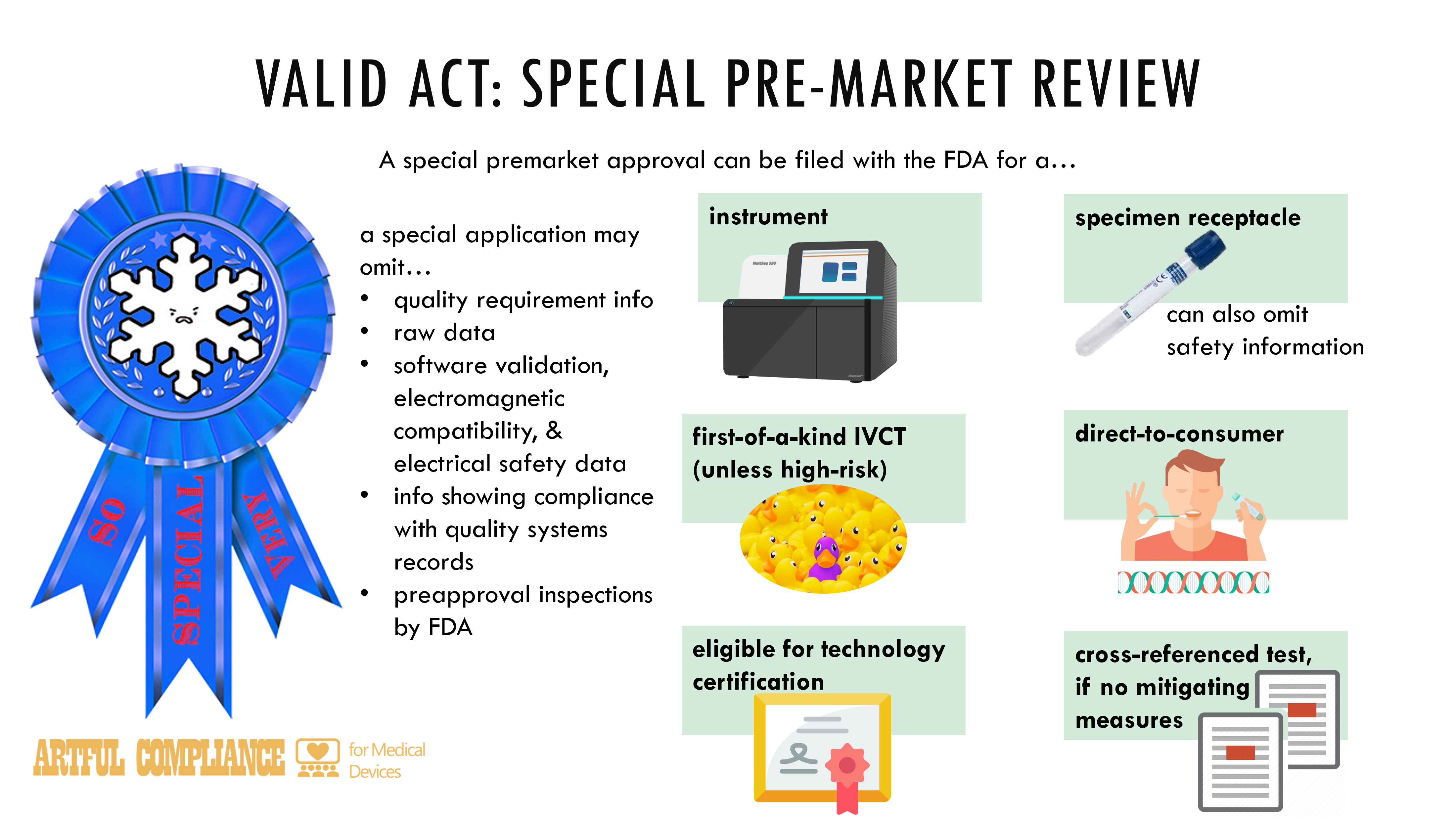
“(1) IN GENERAL.—Any person may file with the Secretary an application for special premarket approval for—
“(A) an instrument;
“(B) a specimen receptacle;
“(C) an in vitro clinical test eligible for a technology certification order under section 587D; or
“(D) a first-of-a-kind test (unless it is a high-risk test), a direct-to-consumer test, or cross-referenced test that does not have mitigating measures.
“(2) APPLICATION CONTENT.—An application under paragraph (1) shall include—
“(A) the information required for applications submitted under subsection (c)(2), except that applications under paragraph (1) need not include—
“(i) quality requirement information; or
“(ii) raw data unless explicitly requested by the Secretary;
“(B) in the case of a specimen receptacle, safety information; and
“(C) data, as applicable, to support software validation, electromagnetic compatibility, and electrical safety, and information demonstrating compliance with maintaining quality systems documentation.
{for special pre-market review:
“(3) INSPECTIONS.—With respect to an application under paragraph (1), preapproval inspections authorized by an employee of the Food and Drug Administration or a person accredited under section 587P need not occur unless requested by the Secretary
Recent Comments