00083 VALID Act General Laboratory Equipment Exemption
From VALID Act
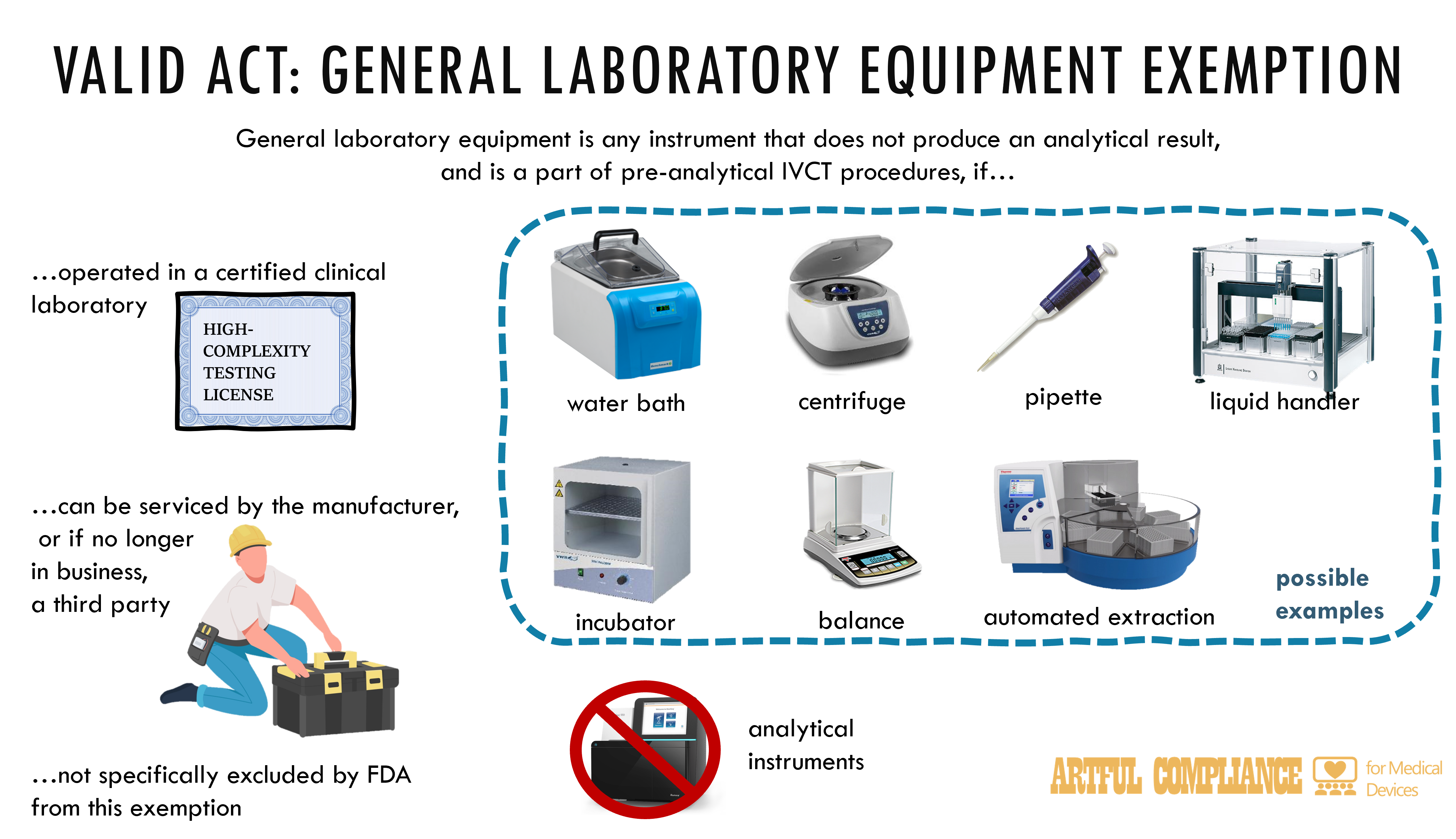
“(o) General laboratory equipment.—Any instrument that does not produce an analytical result, and that functions as a component of pre-analytical procedures related to in vitro clinical tests, is not subject to the requirements of this subchapter, provided that—
“(1) the instrument is operating in a clinical laboratory that is certified under section 353 of the Public Health Service Act; and
“(2) the instrument can be serviced by the manufacturer of such instrument or, if that manufacturer is no longer in business, a third party with the ability to service such instrument.
“(q) General exemption authority.—The Secretary may, by order published in the Federal Register following notice and an opportunity for comment, exempt a class of persons from any section under this subchapter upon a finding that such exemption is appropriate for the protection of the public health and other relevant considerations.
Recent Comments