00082 VALID Act Buying and Selling IVCTs and Notifications
From VALID Act
“(n) Transfer or sale of In vitro clinical tests.—
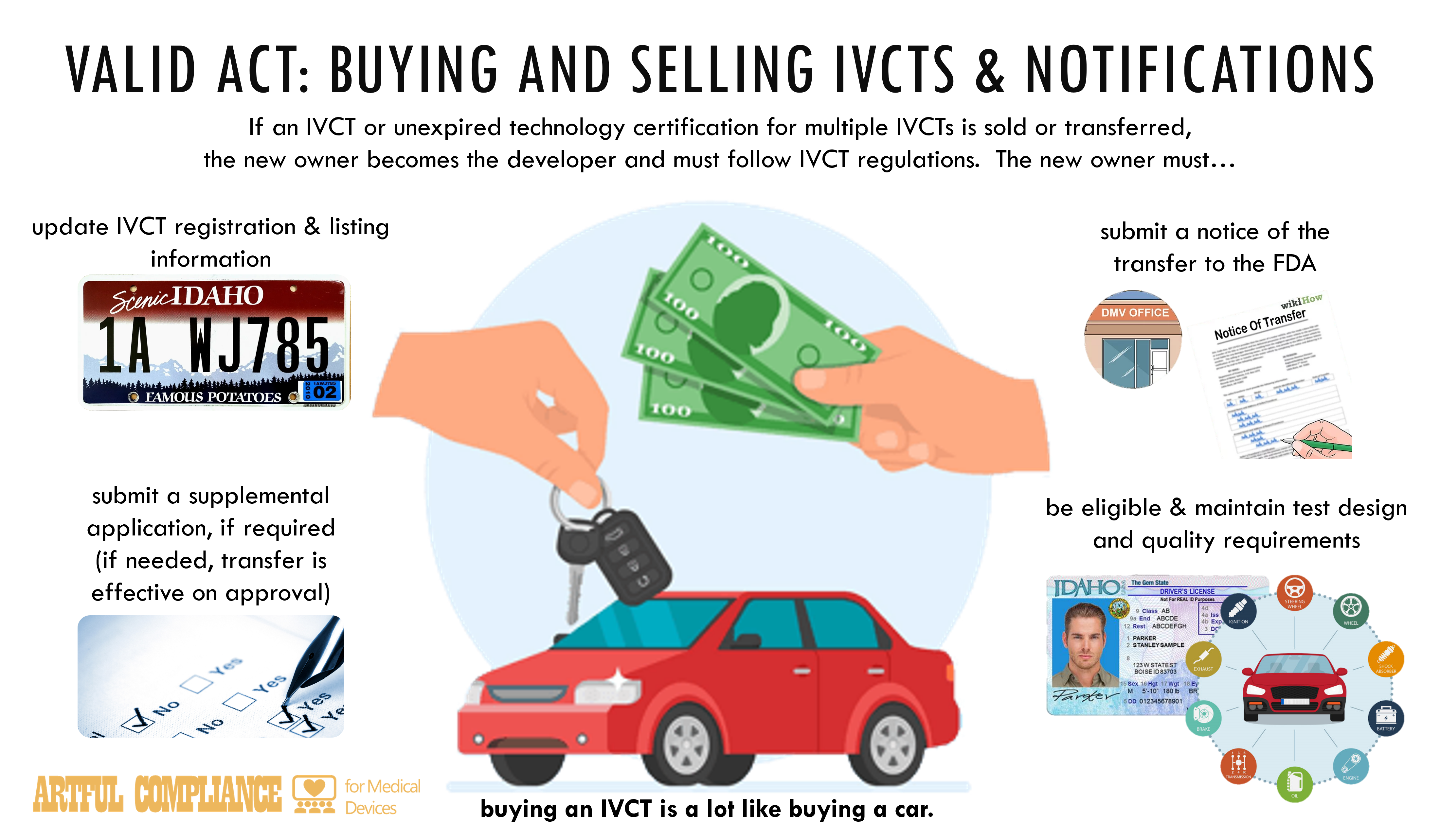
“(1) TRANSFER AND ASSUMPTION OF REGULATORY OBLIGATIONS.—If ownership of an in vitro clinical test is sold or transferred in such manner that the developer transfers the regulatory submissions and obligations applicable under this subchapter with respect to the test, the transferee or purchaser becomes the developer of the test and shall have all regulatory obligations applicable to such a test under this subchapter. The transferee or purchaser shall update the registration and listing information under section 587I for the in vitro clinical test.
“(2) TRANSFER OR SALE OF PREMARKET APPROVAL.—
“(A) NOTICE REQUIRED.—If a developer of an in vitro clinical test transfers or sells the approval of the in vitro clinical test, the transferor or seller shall—
“(i) submit a notice of the transfer or sale to the Secretary and update the registration and listing information under section 587I for the in vitro clinical test; and
“(ii) submit a supplemental application if required under section 587B(h).
“(B) EFFECTIVE DATE OF APPROVAL TRANSFER.—A transfer or sale described in subparagraph (A) shall become effective upon completion of a transfer or sale described in paragraph (1) or the approval of a supplemental application under section 587B(h) if required, whichever is later. The transferee or purchaser shall update the registration and listing information under section 587I for the in vitro clinical test within 15 calendar days of the effective date of the transfer or sale.
“(3) TRANSFER OR SALE OF TECHNOLOGY CERTIFICATION.—
“(A) REQUIREMENTS FOR TRANSFER OR SALE OF TECHNOLOGY CERTIFICATION.—An unexpired technology certification can be transferred or sold if the transferee or purchaser—
“(i) is an eligible person under section 587D(b)(1); and
“(ii) maintains, upon such transfer or sale, the site, test design and quality requirements, processes and procedures under the scope of technology certification, and scope of the technology certification identified in the applicable technology certification order.
“(B) NOTICE REQUIRED.—If a developer of an in vitro clinical test transfers or sells a technology certification order that has not expired, the transferor or seller shall submit a notice of the transfer or sale to the Secretary and shall update the registration and listing information under section 587I for all in vitro clinical tests covered by the technology certification.
“(C) EFFECTIVE DATE OF TECHNOLOGY CERTIFICATION TRANSFER.—The transfer of a technology certification shall become effective upon completion of a transfer or sale described in subparagraph (A). The transferee or purchaser shall update the registration and listing information under section 587I for the in vitro clinical test within 30 calendar days of the effective date of the technology certification transfer.
“(D) NEW TECHNOLOGY CERTIFICATION REQUIRED.—If the requirements of subparagraph (A)(ii) are not met, the technology certification order may not be transferred and the transferee or purchaser of an in vitro clinical test is required to submit an application for technology certification and obtain a technology certification order prior to offering the test for clinical use.
Recent Comments