00067 Public Health Surveillance Exemption
“(i) Public health surveillance activities.—
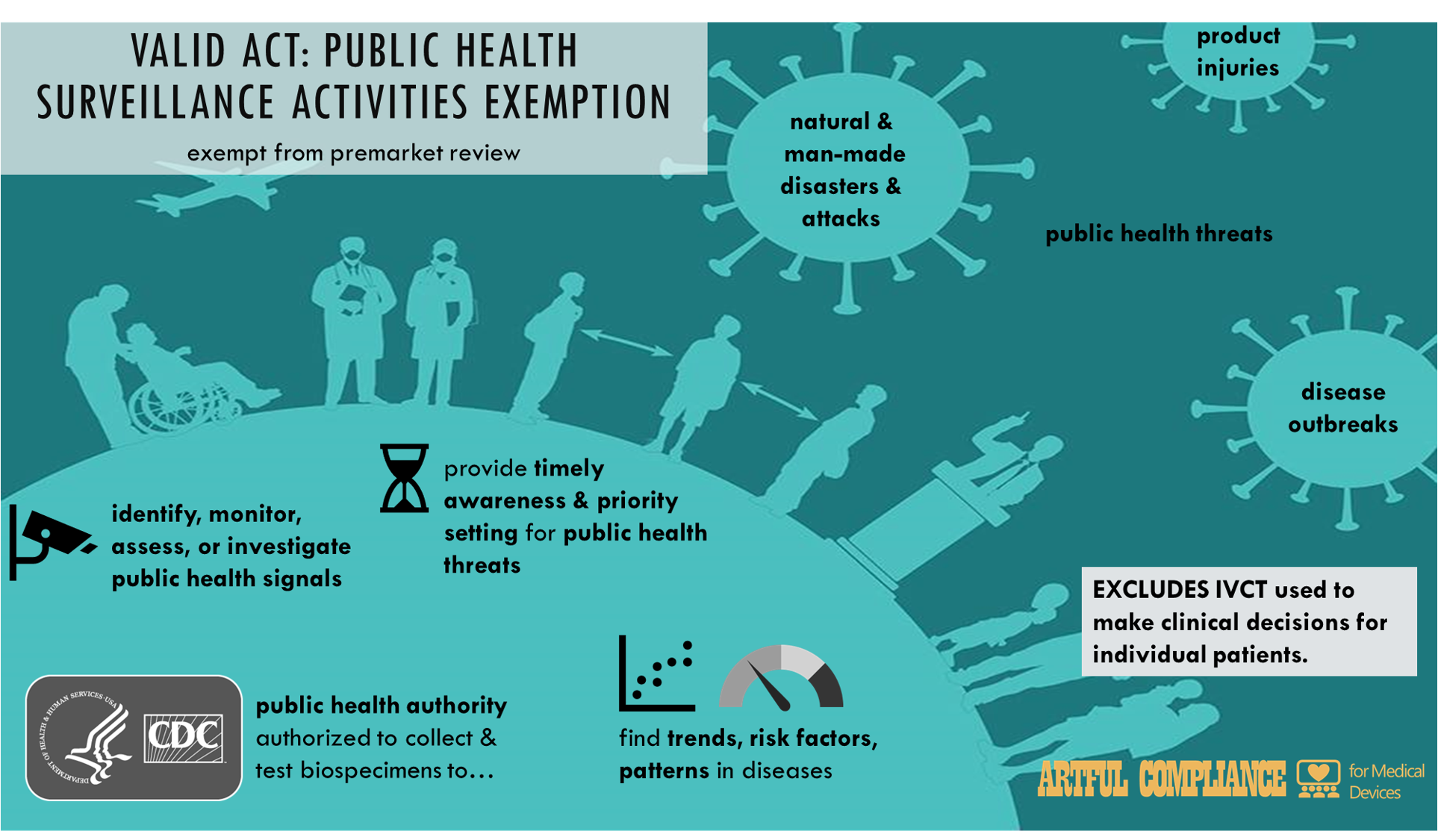
“(1) IN GENERAL.—The provisions of this subchapter shall not apply to a test intended by the developer to be used solely for public health surveillance activities, including the collection and testing of information or biospecimens, conducted, supported, requested, ordered, required, or authorized by a public health authority.
“(2) LIMITATION.—The public health surveillance activities described in paragraph (1)—
“(A) are limited to activities necessary to allow a public health authority to identify, monitor, assess, or investigate potential public health signals, onsets of disease outbreaks, or conditions of public health importance (including trends, risk factors, patterns in diseases, and increases in injuries from using consumer products); and
“(B) include activities associated with providing timely situational awareness and priority setting during the course of a threat to the public health (including natural or man-made disasters and deliberate attacks on the United States).
“(3) EXCLUSION.—An in vitro clinical test is not excluded from the provisions of this subchapter if such test is intended for use in making clinical decisions for individual patients.
Recent Comments