00065 Custom and Low-Volume Test Exemption
“(h) Custom tests and low-Volume tests.—An in vitro clinical test is exempt from premarket review under section 587B, the quality requirements under section 587J, and the notification requirements under section 587I, and may be lawfully marketed subject to the other applicable requirements of this Act, if—
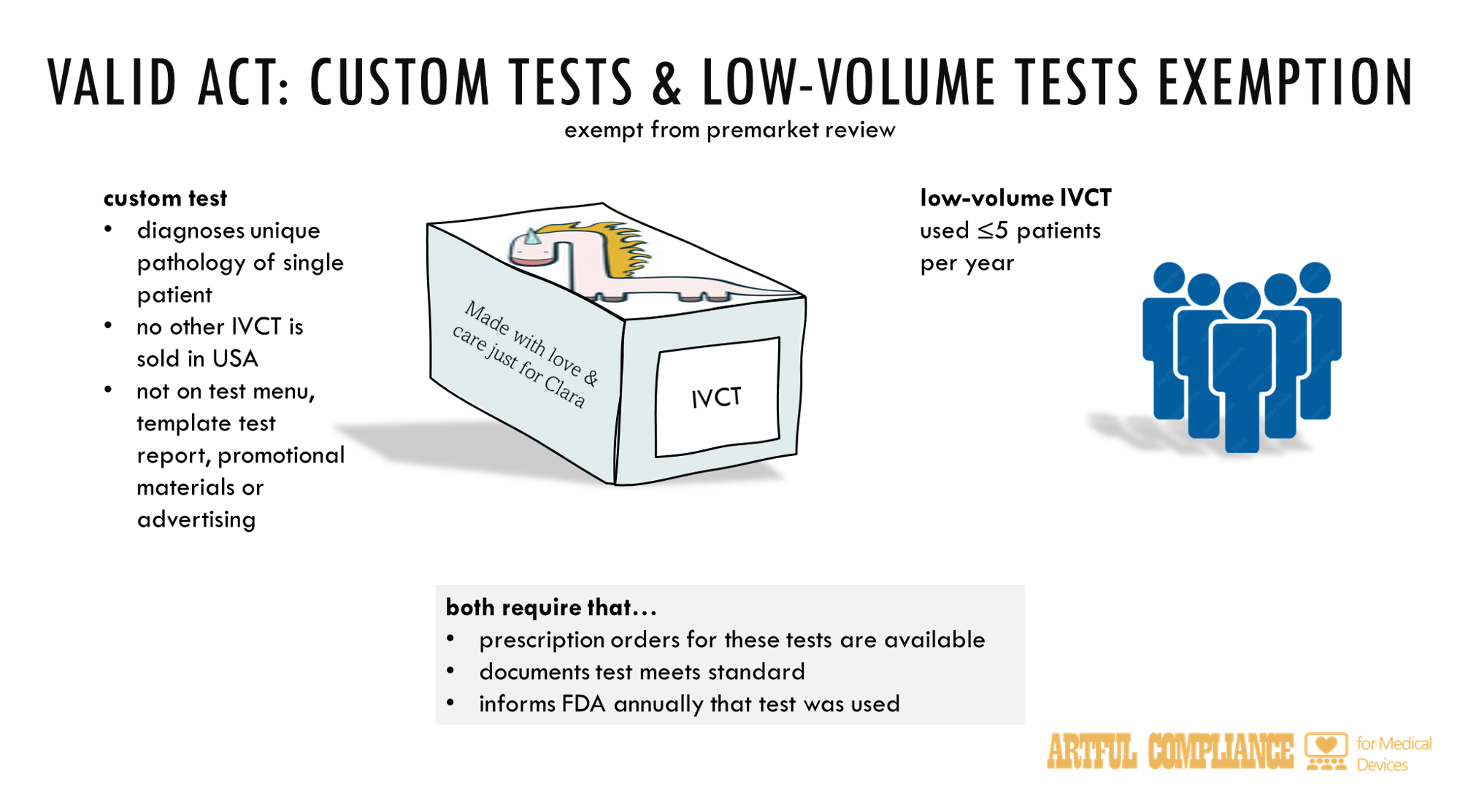
“(1) such in vitro clinical test—
“(A) is a low-volume test performed in a laboratory in which it was developed or developed in a laboratory within the same corporate organization with the laboratory in which such test is performed and is administered to no more than 5 patients per year, unless otherwise determined by the Secretary; or
“(B) is a custom test developed or modified to diagnose a unique pathology or physical condition of a specific patient for which no other in vitro clinical test is commercially available in the United States, and is—
“(i) not intended for use with respect to other patients; and
“(ii) after the development of the custom test, not included in any test menu, template test report, or other promotional materials, and not otherwise advertised; and
“(2) the developer of the test—
“(A) maintains documentation demonstrating that such test meets and continues to meet the applicable criteria described in paragraph (1);
“(B) makes such documentation, such as a prescription order requesting the custom test for an individual patient, available to the Secretary upon request; and
“(C) informs the Secretary, on an annual basis, in a manner prescribed by the Secretary by guidance, that such test was introduced into interstate commerce.
Recent Comments