00064 Humanitarian Test Exemption
VALID Act “(g) Humanitarian test exemption.—
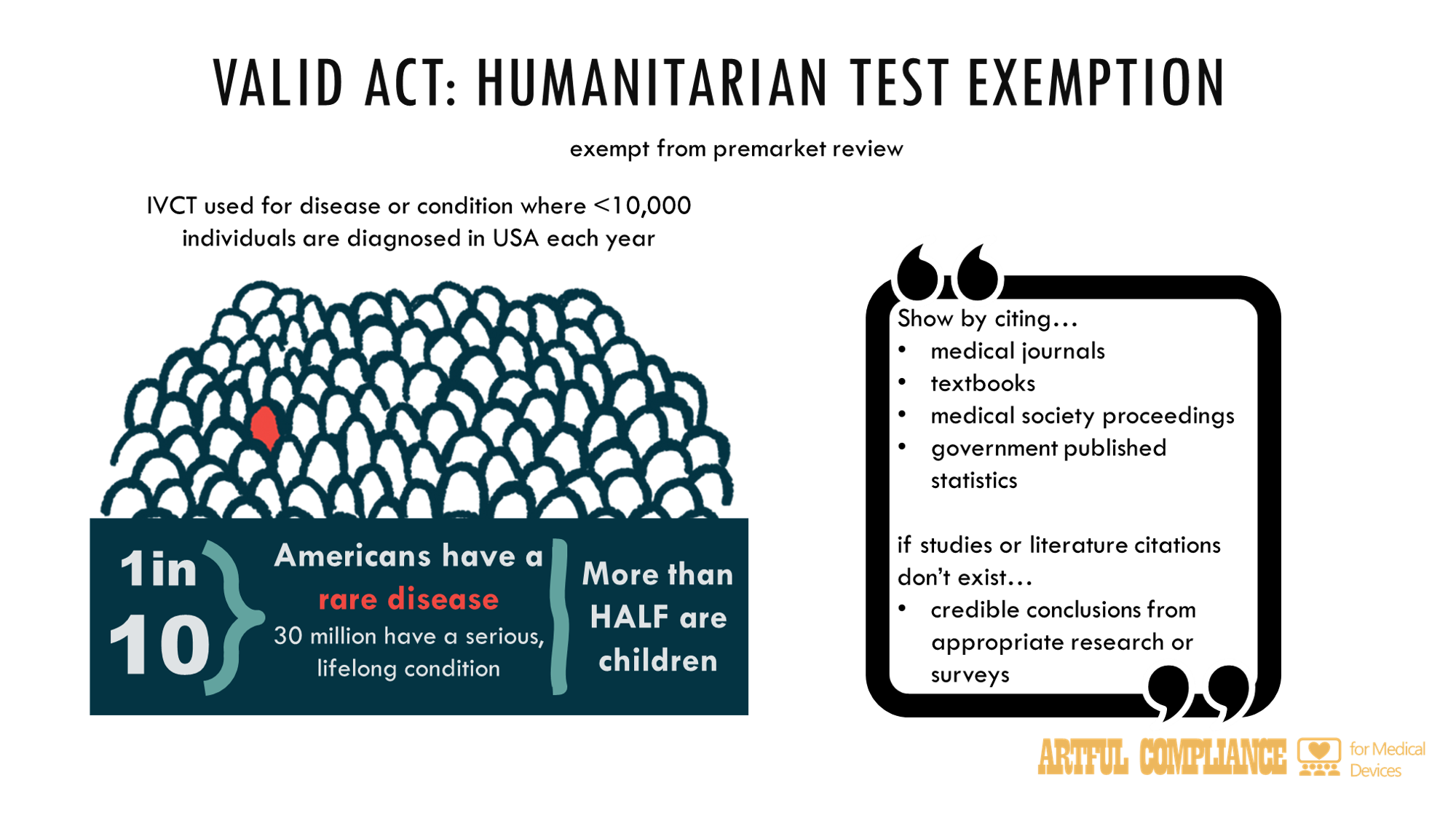
“(1) IN GENERAL.—An in vitro clinical test is exempt from premarket review under section 587B and may be lawfully marketed subject to the other applicable requirements of this Act, if—
“(A) such in vitro clinical test is intended for use for a disease or condition for which no more than 10,000 (or such other number determined by the Secretary) individuals would be subject to negative or positive diagnosis by such test in the United States per year; and
“(B) the developer of the test—
“(i) maintains documentation (which may include literature citations in specialized medical journals, textbooks, specialized medical society proceedings, governmental statistics publications, or, if no such studies or literature citations exist, credible conclusions from appropriate research or surveys) demonstrating that such test meets and continues to meet the criteria described in this paragraph; and
“(ii) makes such documentation available to the Secretary upon request.
Recent Comments