00060 VALID Act Emergency Use Authorization
“(5) EMERGENCY USE.—
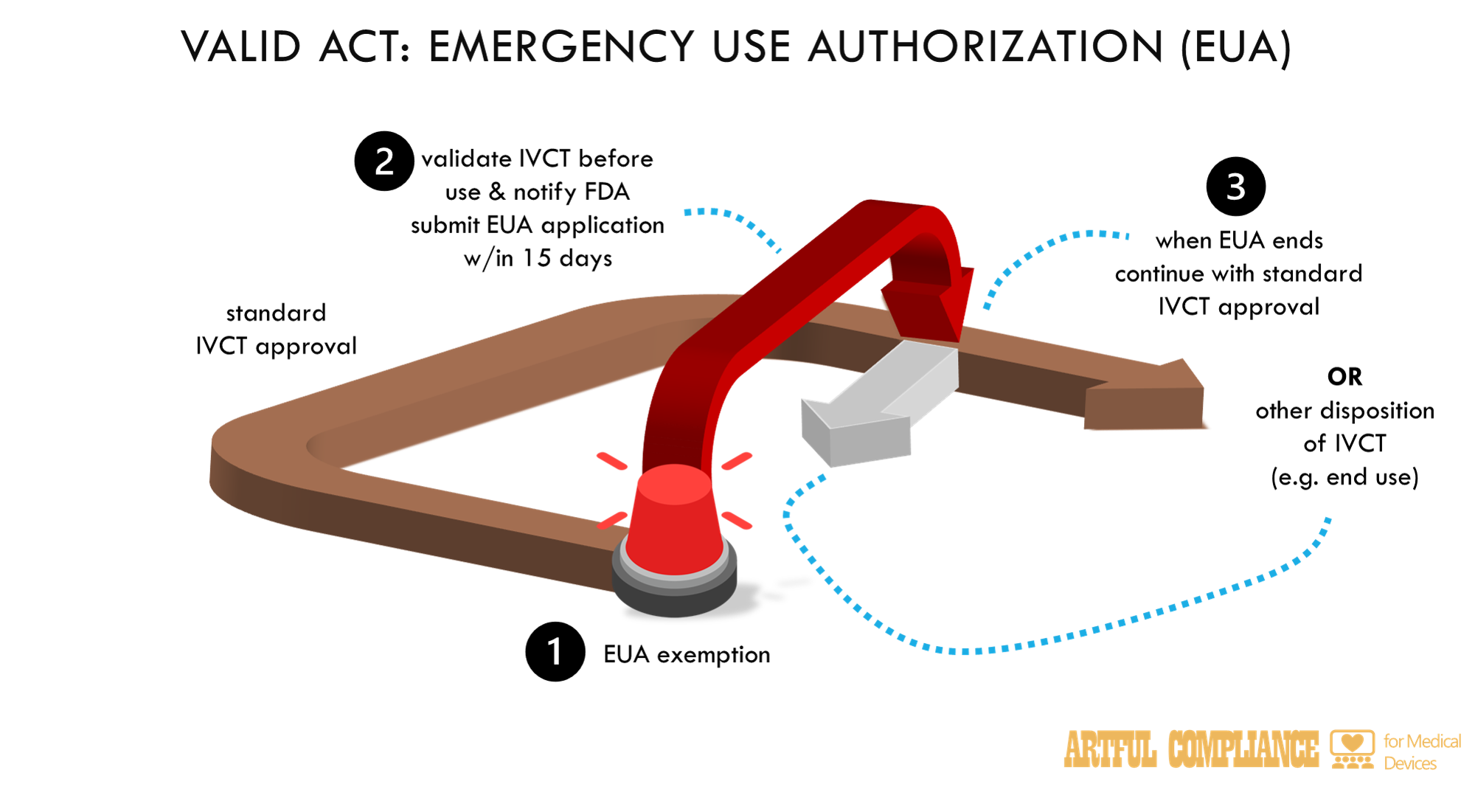
“(A) IN GENERAL.—In the case of a determination under section 319(a) of the Public Health Service Act or a declaration under section 564(b) of this Act, an in vitro clinical test is exempt from the requirements of this subchapter and may be lawfully marketed in accordance with subparagraph (B).
“(B) CRITERIA.—An in vitro clinical test is exempt from the requirements of this subchapter and may be lawfully marketed in accordance with the exemption described in subparagraph (A) if—
“(i) such test—
“(I) is submitted for emergency use authorization under section 564(b); or
“(II) is developed and used in laboratories for which a certificate is in effect under section 353 of the Public Health Service Act to conduct high-complexity testing and the developer; and
“(ii) the developer—
“(I) validates such in vitro clinical test prior to use;
“(II) notifies the Secretary of the assay validation; and
“(III) submits an emergency use authorization application under section 564 within 15 calendar days of marketing the test.
“(C) DISPOSITION OF PRODUCT.—With respect to a previously unapproved in vitro clinical test or an in vitro clinical test with an unapproved use, for which an emergency use authorization under section 564(b) ceases to be effective, the Secretary shall consult with the manufacturer of such product with respect to the appropriate disposition of the product.
“(D) STREAMLINING OF APPLICATION REVIEW.—A developer may include any data or information already submitted to the Secretary within the emergency use authorization as a part of a premarket application under section 587B or a technology certification application under section 587D.
Recent Comments