00059 VALID Act: Developer Disagreement about IVCT Exemption
“(4) SPECIAL RULE.—
“(A) PREMARKET REVIEW APPLICABLE.—Notwithstanding the exemptions from premarket review under section 587B set forth in subsections (b), (c), (d), (e), (f), (g), (h), (j), and (k) of such section, an in vitro clinical test (including any article for taking or deriving specimens) shall be subject to the requirements of section 587B if the Secretary determines, in accordance with subparagraph (B), that—
“(i) (I) there is insufficient valid scientific evidence to support the analytical validity or the clinical validity of such in vitro clinical test; and
“(II) such in vitro clinical test is being offered by its developer with materially deceptive or fraudulent analytical or clinical claims;
“(ii) it is reasonably possible that such in vitro clinical test will cause serious adverse health consequences; or
“(iii) in the case of specimen receptacles, there is sufficient valid scientific evidence indicating that a specimen receptacle did not perform as intended, will not support the analytical validity of tests with which it is used, or as applicable, is not safe for use.
“(B) PROCESS.—
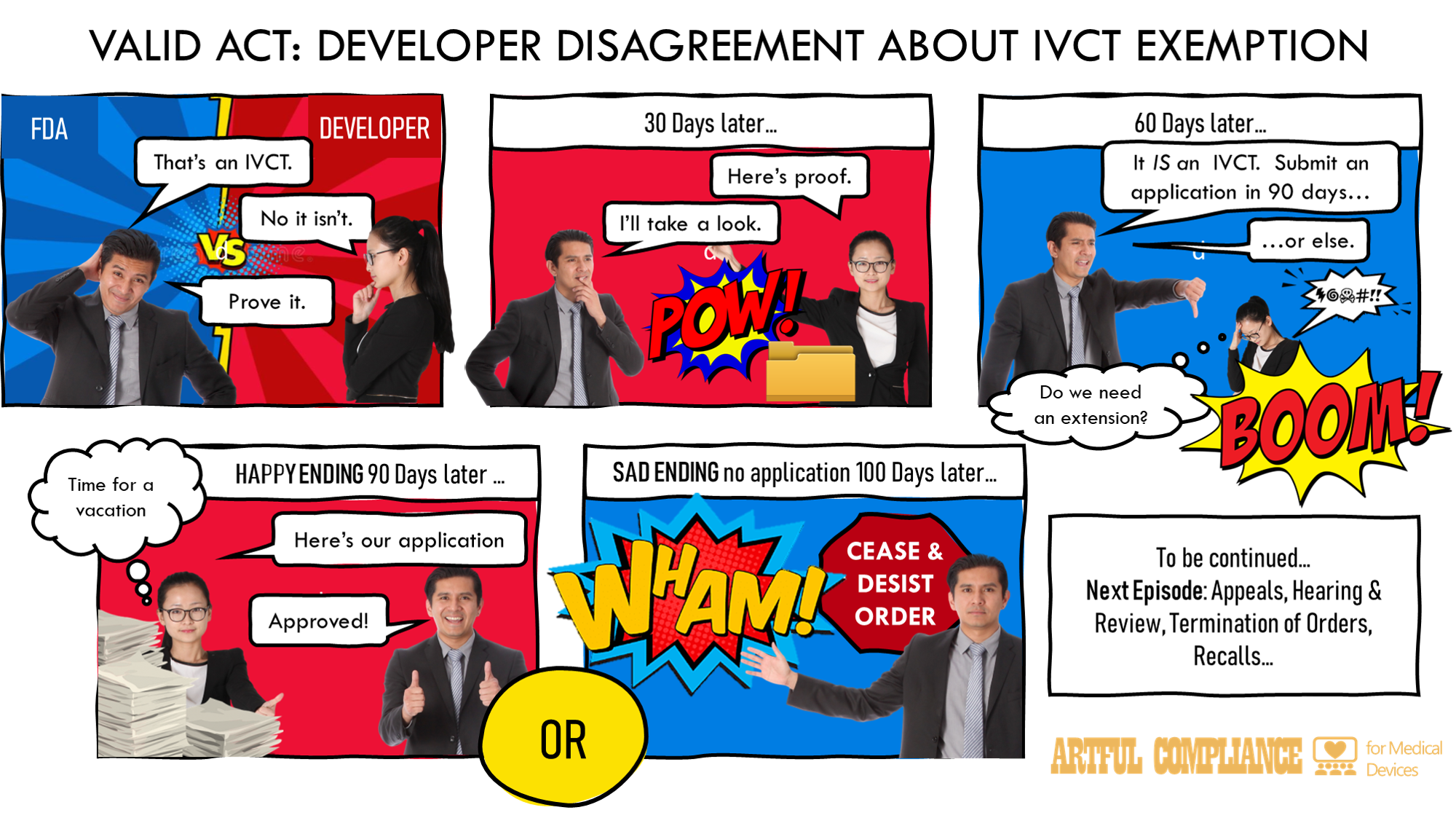
“(i) REQUEST FOR INFORMATION.—If the Secretary has valid scientific evidence indicating that the criteria listed in subparagraph (A) apply to an in vitro clinical test, the Secretary may request that the developer of the test submit information—
“(I) pertaining to such criteria; and
“(II) establishing the basis for any claimed exemption from premarket review.
“(ii) DEADLINE FOR SUBMITTING INFORMATION.—The developer of an in vitro clinical test shall submit the information requested pursuant to clause (i) within 30 days of receipt of such request.
“(iii) REVIEW DEADLINE.—Upon receiving a submission under clause (ii), the Secretary shall—
“(I) review the submitted information within 60 calendar days of such receipt; and
“(II) determine whether the criteria listed in subparagraph (A) apply to the in vitro clinical test.
“(iv) PREMARKET REVIEW REQUIRED.—
“(I) IN GENERAL.—If the Secretary finds that the criteria listed in subparagraph (A) apply to the in vitro clinical test, the developer shall—
“(aa) promptly, and not later than 90 days after the date of receipt of such information, submit an application for premarket review of the test under section 587B; or
“(bb) cease to market the test.
“(II) EXTENSION.—The Secretary may grant an extension to a developer of the 90-day time period under subclause (I)(aa), as appropriate.
“(v) CONTINUED MARKETING.—During the period beginning on the date of a request for information under clause (ii) and ending on the date of the disposition of an application for premarket review of the in vitro clinical test under section 587B, the developer of the test may continue to market the test for clinical use, unless the Secretary issues an order to the developer under clause (vi) to immediately cease distribution of the test.
“(vi) ORDER TO CEASE DISTRIBUTION.—
“(I) IN GENERAL.—If the developer of an in vitro clinical test fails to submit an application for premarket review of the test by the deadline applicable under clause (iv), or the Secretary finds that the criteria listed in subparagraph (A) apply to an in vitro clinical test and that it is in the best interest of the public health, the Secretary may issue an order, within 10 calendar days of the applicable deadline or finding by the Secretary, requiring the developer of such in vitro clinical test, and any other appropriate person (including a distributor or retailer of the in vitro clinical test) to immediately—
“(aa) cease distribution of the test pending approval of an application for premarket review of the test under section 587B; and
“(bb) notify health professionals and other user facilities of the order to cease distribution and advise health care professionals to cease use of such in vitro clinical test.
“(II) HEARING AND REVIEW.—An order under subclause (I) shall provide the person subject to the order with an opportunity for an informal hearing, to be held not later than 10 days after the date of the issuance of the order, on the actions required by the order and on whether the order should be amended to require a recall of such in vitro clinical test. If, after providing an opportunity for such a hearing, the Secretary determines that inadequate grounds exist to support the actions required by the order, the Secretary shall terminate the order within 30 days of the hearing. Upon terminating an order, the Secretary shall provide written notice of such termination to the developer.
“(vii) AMENDMENT TO REQUIRE RECALL.—If the Secretary determines that an order issued under clause (vi) should be amended to include a recall of the in vitro clinical test with respect to which the order was issued, the Secretary shall amend the order to require a recall. In such amended order, the Secretary shall specify a timeframe in which the in vitro clinical test recall will occur and shall require periodic reports to the Secretary describing the progress of the recall. Upon termination of the recall, the Secretary shall provide written notice of such termination to the developer.
“(viii) EFFECT OF TEST APPROVAL.—Any order issued under this paragraph with respect to an in vitro clinical test shall cease to be in effect if such test is granted approval under section 587B, provided that the in vitro clinical test is developed and offered for clinical use in accordance with such approval.
Recent Comments