00053 VALID Act; Cross-Referenced Test
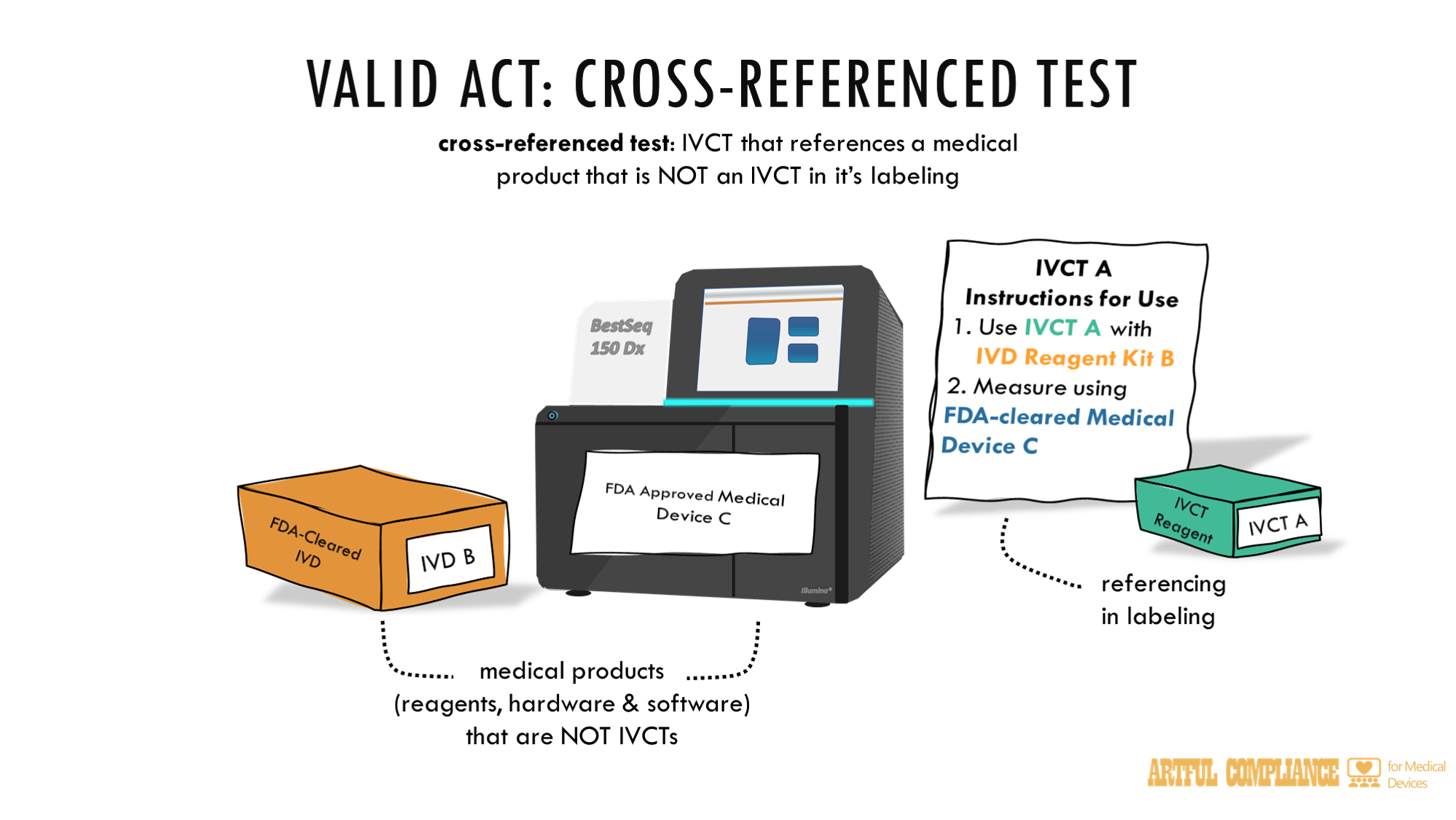
CROSS-REFERENCED TEST.—The term ‘cross-referenced test’ means an in vitro clinical test that references in its labeling the name or intended use of another medical product that is not an in vitro clinical test.
“(2) CROSS-REFERENCED TESTS.—In order to be eligible for an exemption under this subsection, the developer of a cross-referenced test shall submit a request under section 587H for informal feedback.
Recent Comments