00051 VALID Act: IVCT Technology
From VALID Act Definitions
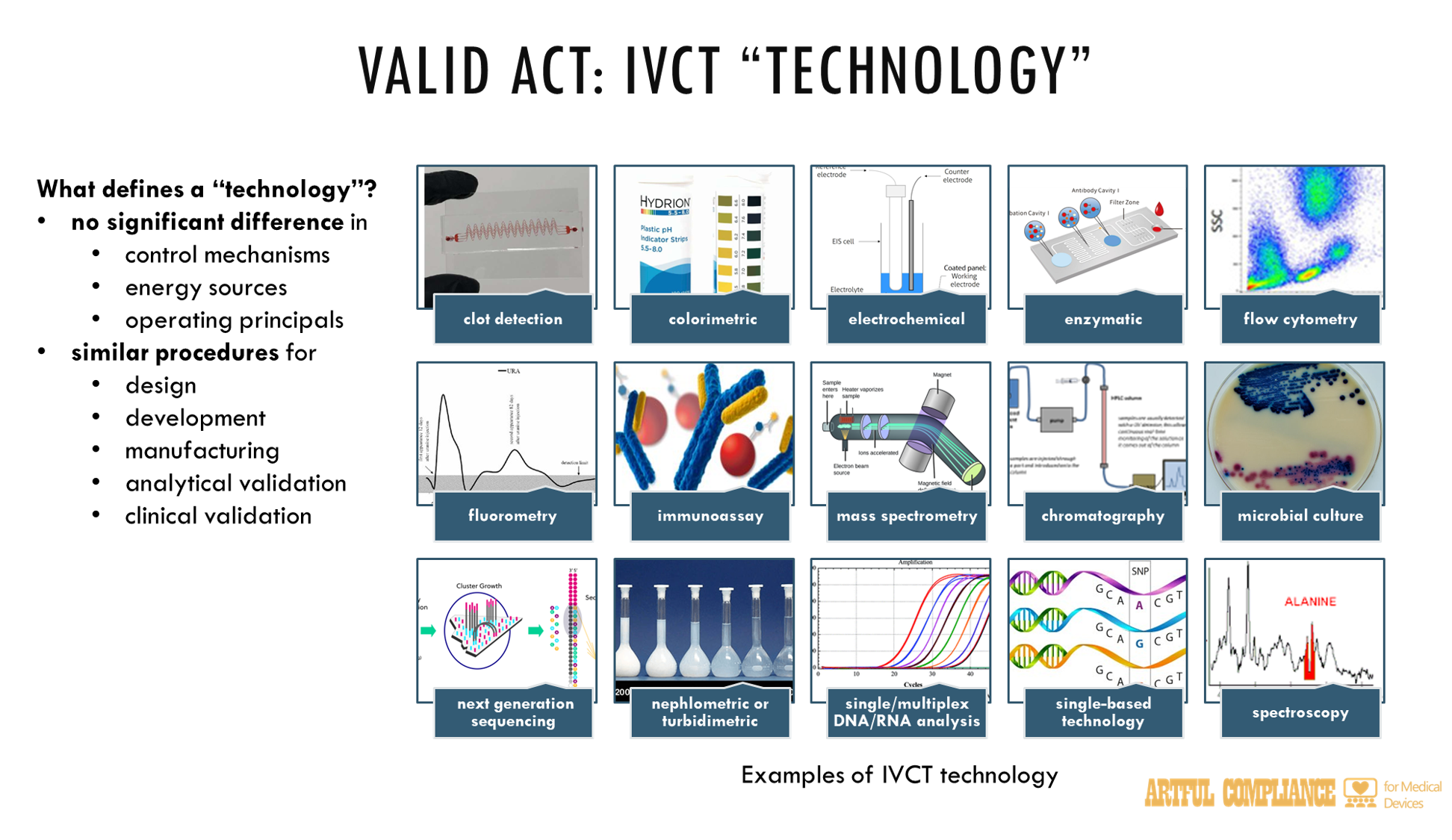
“(17) TECHNOLOGY.—The term ‘technology’—
“(A) means a developer’s grouping of in vitro clinical tests that do not significantly differ in control mechanisms, energy sources, or operating principals and for which design, development, and manufacturing, including analytical and clinical validation as applicable, of the tests would be addressed in a similar manner or through similar procedures; and
“(B) may include clot detection, colorimetric (non-immunoassay), electrochemical (non-immunoassay), enzymatic (non-immunoassay), flow cytometry, fluorometry (non-immunoassay), immunoassay, mass spectrometry or chromatography (such as HPLC), microbial culture, next generation sequencing (also known as ‘NGS’), nephlometric or turbidimetric (non-immunoassay), singleplex or multiplex non-NGS nucleic acid analysis, single-based technology, spectroscopy, and any other technology, as the Secretary determines appropriate.
Recent Comments