00050 VALID Act: Instrument and Instrument Family
From VALID Act Definitions section
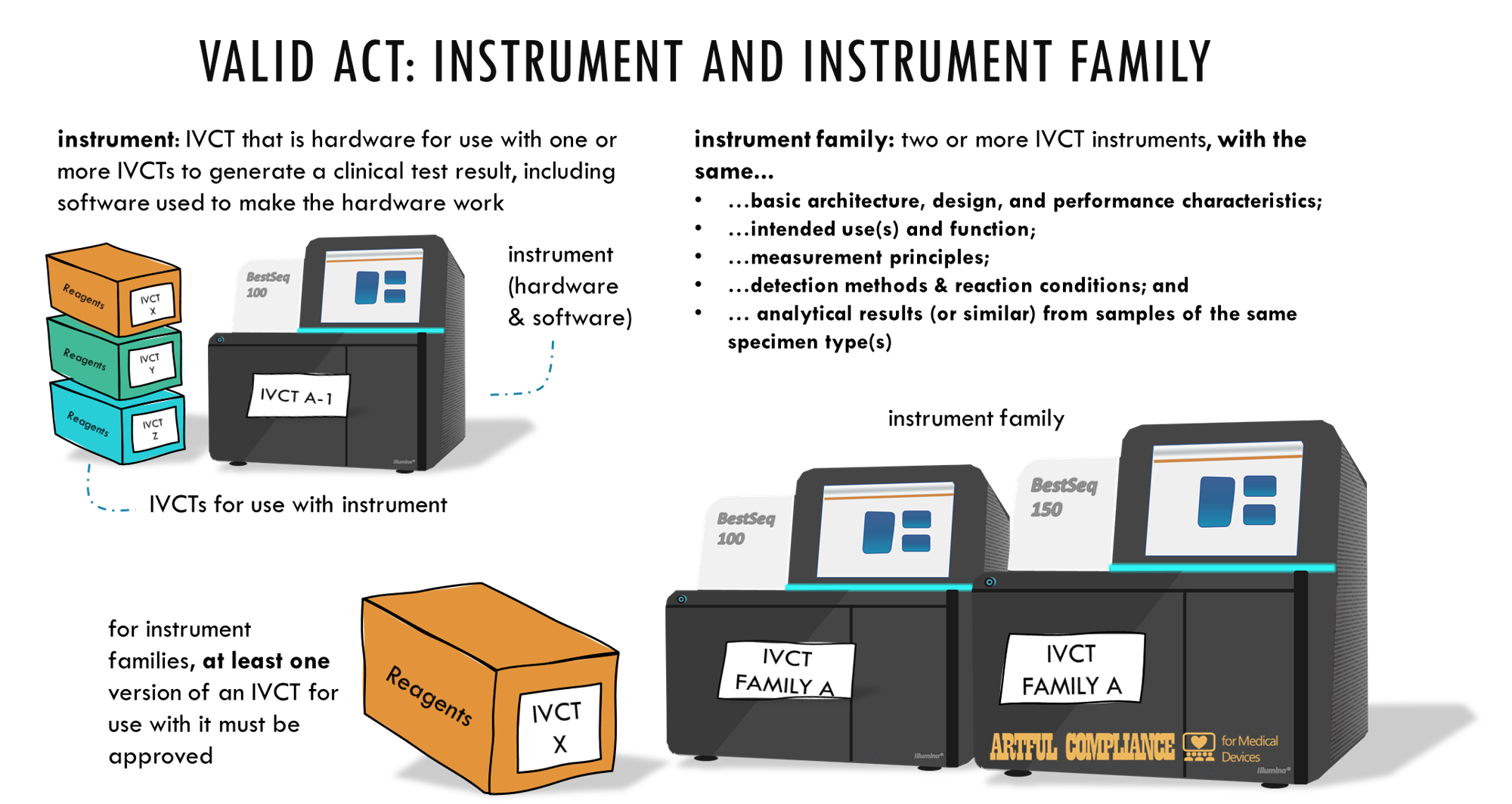
“(11) INSTRUMENT.—The term ‘instrument’ means an in vitro clinical test that is hardware intended by the hardware’s developer to be used with one or more in vitro clinical tests to generate a clinical test result, including software used to effectuate the hardware’s functionality.
“(12) INSTRUMENT FAMILY.—The term ‘instrument family’ means more than one instrument for which the developer demonstrates and documents, with respect to all such instruments, that all—
“(A) have the same basic architecture, design, and performance characteristics, such as tolerance limits and signal range;
“(B) have the same intended use or uses and function;
“(C) share the same measurement principles, detection methods, and reaction conditions; and
“(D) produce the same or similar analytical results from samples of the same specimen type or types.
“(p) Instrument families.—In the case of an instrument family, premarket approval under section 587B(d) of one version of the in vitro clinical test is required, and previous and updated versions of the same test within such instrument family shall be deemed to be subject to the approval pursuant to that section, unless the Secretary determines otherwise, as set forth in guidance.
“SEC. 587B. Premarket review.
“(e) Instrument family.—When an in vitro clinical test has been approved, or is otherwise legally marketed, for use on a specific approved or legally marketed instrument within an instrument family, a submission under this section shall not be required for that in vitro clinical test in order for it to be used on a new instrument within that instrument’s family.
Recent Comments