Risk Classification
In the US, IVDs have a three-tier risk classed (I, II, III). Globally, a four tier is most common (e.g. A, B, C, D). But IVCTs have only two classes: low or high risk, based on the likeliness of harm from an inaccurate result. For a subset of high-risk IVCTs for which FDA will create risk-reducing measures (e.g., special controls requirements for test development, labeling, validation, etc.).
From the VALID Act, “SEC. 587A. Applicability.
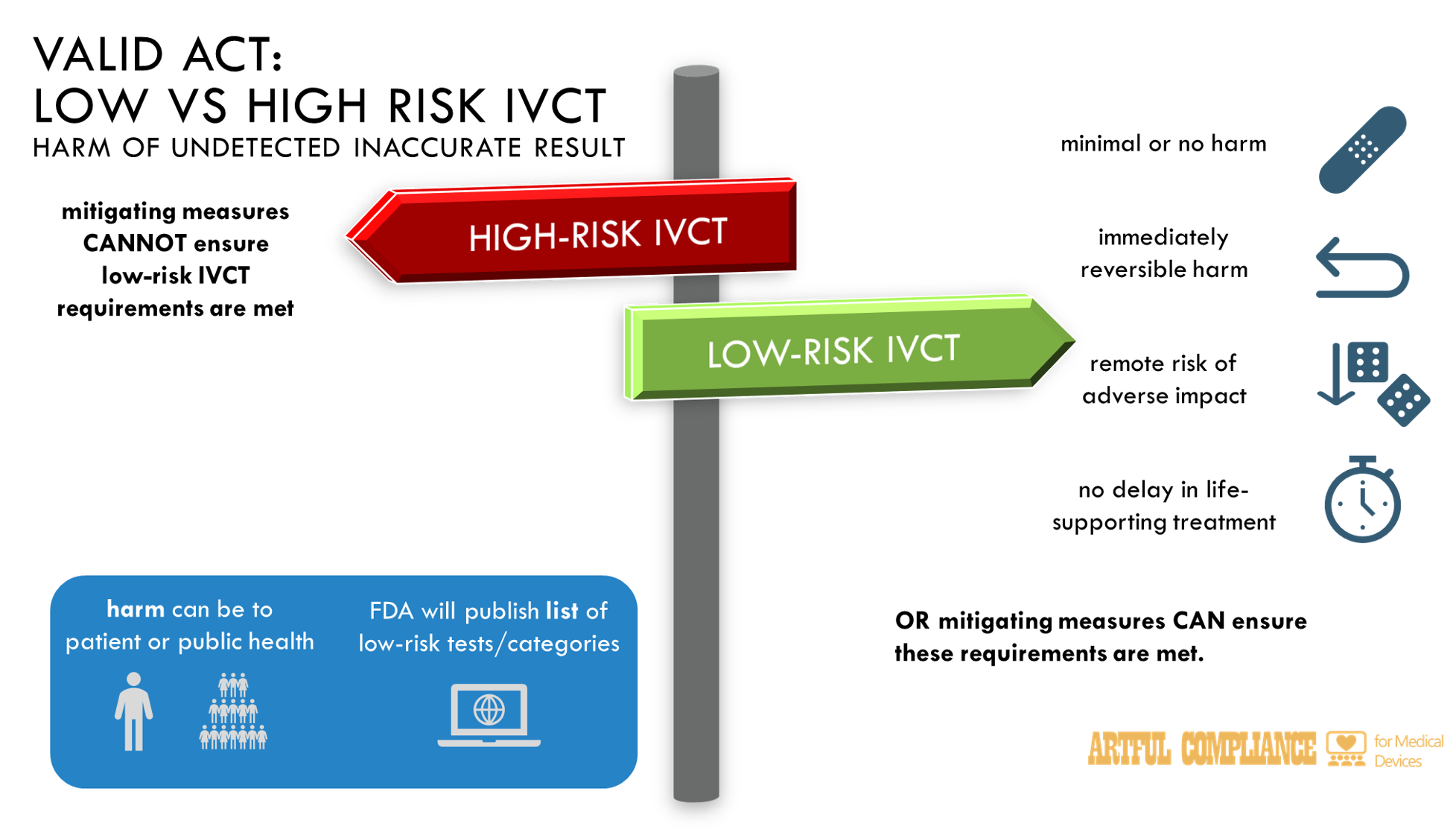
“(14) LOW-RISK.—The term ‘low-risk’, with respect to an in vitro clinical test or category of in vitro clinical tests, means that an undetected inaccurate result from such in vitro clinical test, or such category of in vitro clinical tests, when used as intended by the developer—
“(A) would cause minimal or no harm, or minimal or no disability, or immediately reversible harm, or would lead to only a remote risk of adverse patient impact or adverse public health impact, taking into account the degree to which the technology for the intended use of an in vitro clinical test or category of tests is well-characterized and the criteria for performance of the test or category of tests are well-established for the intended use, the clinical circumstances under which the in vitro clinical test or category of tests is used, and the availability of other tests (such as confirmatory or adjunctive tests); or
“(B) would cause a serious adverse health consequence, harm that is reversible, a delay in necessary treatment that is not life-supporting or life-sustaining, or would lead to a serious risk of adverse patient experience or adverse public health impact, but applied mitigating measures have the capacity to ensure the test meets the standard described in subparagraph (A).
(9) HIGH-RISK.—
“(A) IN GENERAL.—Subject to subparagraph (B), the term ‘high-risk’, with respect to an in vitro clinical test or category of in vitro clinical tests—
“(i) means that, when used as intended by the developer, an undetected inaccurate result from such test or category—
“(I) presents unreasonable risk for serious or irreversible harm or death to a patient or patients, or would otherwise cause serious harm to the public health; or
“(II) is potentially likely to result in the absence, significant delay, or discontinuation of life-supporting or life-sustaining medical treatment; and
“(ii) shall account for the degree to which the technology for the intended use of an in vitro clinical test or tests is well-characterized and the criteria for performance of the test or tests are well-established for the intended use, the clinical circumstances under which the in vitro clinical test is used, and the availability of other tests (such as confirmatory or adjunctive tests).
(B) EXCEPTION.—The term ‘high-risk’ does not include an in vitro clinical test described in subparagraph (A) if—
“(i) mitigating measures are established to prevent, detect, or otherwise mitigate the risk of inaccurate results as described in subparagraph (A), or
“(ii) an exemption from the definition of such term applies under section 587A.
“SEC. 587A. Applicability.
“(e) Low-Risk tests.—
“(1) EXEMPTION.—An in vitro clinical test is exempt from premarket review under section 587B and may be lawfully marketed subject to the other applicable requirements of this Act, including section 587I(b)(6), if such test meets the definition of low-risk under section 587.
“(2) LIST OF LOW-RISK TESTS.—
“(A) IN GENERAL.—The Secretary shall maintain, and make publicly available on the website of the Food and Drug Administration, a list of in vitro clinical tests, and categories of in vitro clinical tests, that are low-risk in vitro clinical tests for purposes of the exemption under this subsection.
“(B) INCLUSION.—The list under subparagraph (A) shall consist of—
“(i) all in vitro clinical tests and categories of in vitro clinical tests that are exempt from premarket review pursuant to subsection (d)(1) or (d)(3); and
“(ii) all in vitro clinical tests and categories of in vitro clinical tests that are designated by the Secretary pursuant to subparagraph (C) as low-risk for purposes of this subsection.
“(C) DESIGNATION OF TESTS AND CATEGORIES.—Without regard to subchapter II of chapter 5 of title 5, United States Code, the Secretary may designate, in addition to the tests and categories described in subparagraph (B)(i), additional in vitro clinical tests, and categories of in vitro clinical tests, as low-risk in vitro clinical tests for purposes of the exemption under this subsection. The Secretary may make such a designation on the Secretary’s own initiative or in response to a request by any person. In making such a designation for a test or category of tests, the Secretary shall consider—
“(i) whether the test, or category of tests, is low-risk; and
“(ii) such other factors as the Secretary determines to be relevant to the protection of the public health.
“(2) HIGH-RISK TEST LIMITATION OR CONDITION.—A high-risk test may be exempt under paragraph (1) from the requirements of this subchapter only if—
“(A) no component or part of such test, including any reagent, is introduced into interstate commerce under the exemption under subsection (b)(1) (relating to components or parts intended for further development), and any article for taking or deriving specimens from the human body used in conjunction with the test remains subject to the requirements of this subchapter; or
“(B) the test has been developed in accordance with the applicable test design and quality requirements under section 587J.
Recent Comments