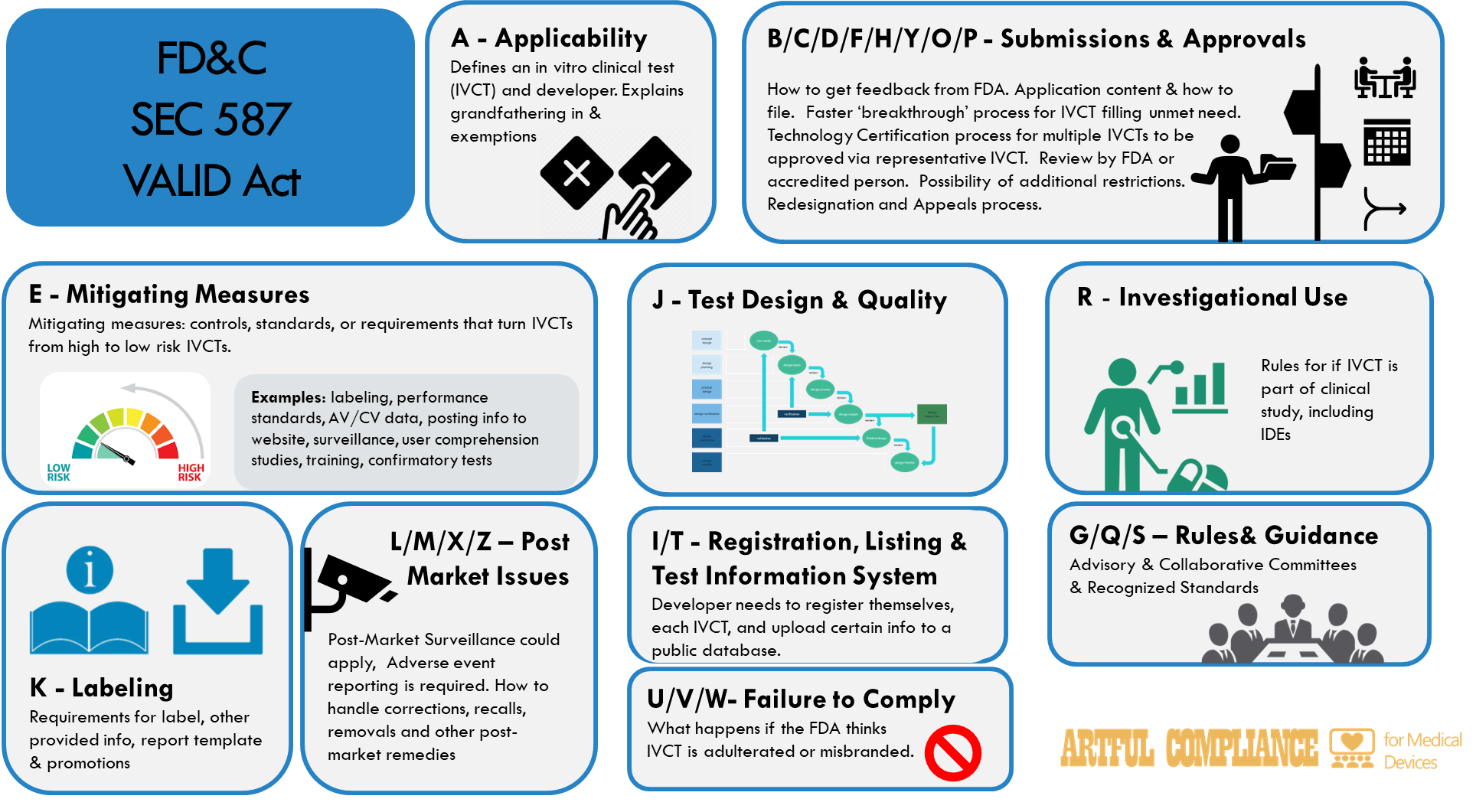
The VALID Act, is a draft Law that describes In Vitro Clinical Test (IVCT). Regulations have not yet been drafted, but are likely to mirror the law.
“Sec. 587A. Applicability. “Sec. 587N. Restricted in vitro clinical tests.
“Sec. 587B. Premarket review. “Sec. 587C. Breakthrough in vitro clinical tests. “Sec. 587D. Technology certification. “Sec. 587F. Regulatory pathway redesignation. “Sec. 587H. Request for informal feedback. “Sec. 587Y. Electronic format for submissions. “Sec. 587O. Appeals. “Sec. 587P. Accredited persons.
“Sec. 587E. Mitigating measures.
“Sec. 587J. Test design and quality requirements.
“Sec. 587K. Labeling requirements.
“Sec. 587X. Postmarket surveillance. “Sec. 587L. Adverse event reporting. “Sec. 587M. Corrections and removals. “Sec. 587Z. Postmarket remedies.
“Sec. 587G. Advisory committees. “Sec. 587Q. Recognized standards. “Sec. 587S. Collaborative communities for in vitro clinical tests.
“Sec. 587R. Investigational use.
“Sec. 587I. Registration and listing. “Sec. 587T. Comprehensive test information system.
“Sec. 587V. Adulteration. “Sec. 587W. Misbranding. “Sec. 587U. Preemption
. Sec. 4. Enforcement and other provisions. Sec. 5. Transition. /Sec. 6. Emergency use authorization. /Sec. 7. Antimicrobial susceptibility tests. /Sec. 8. Combination products./ Sec. 9. Resources.
Recent Comments