
FDC-Section-587-Valid-Act-IVCT-Definition
FD&C Act SEC. 587 Definitions
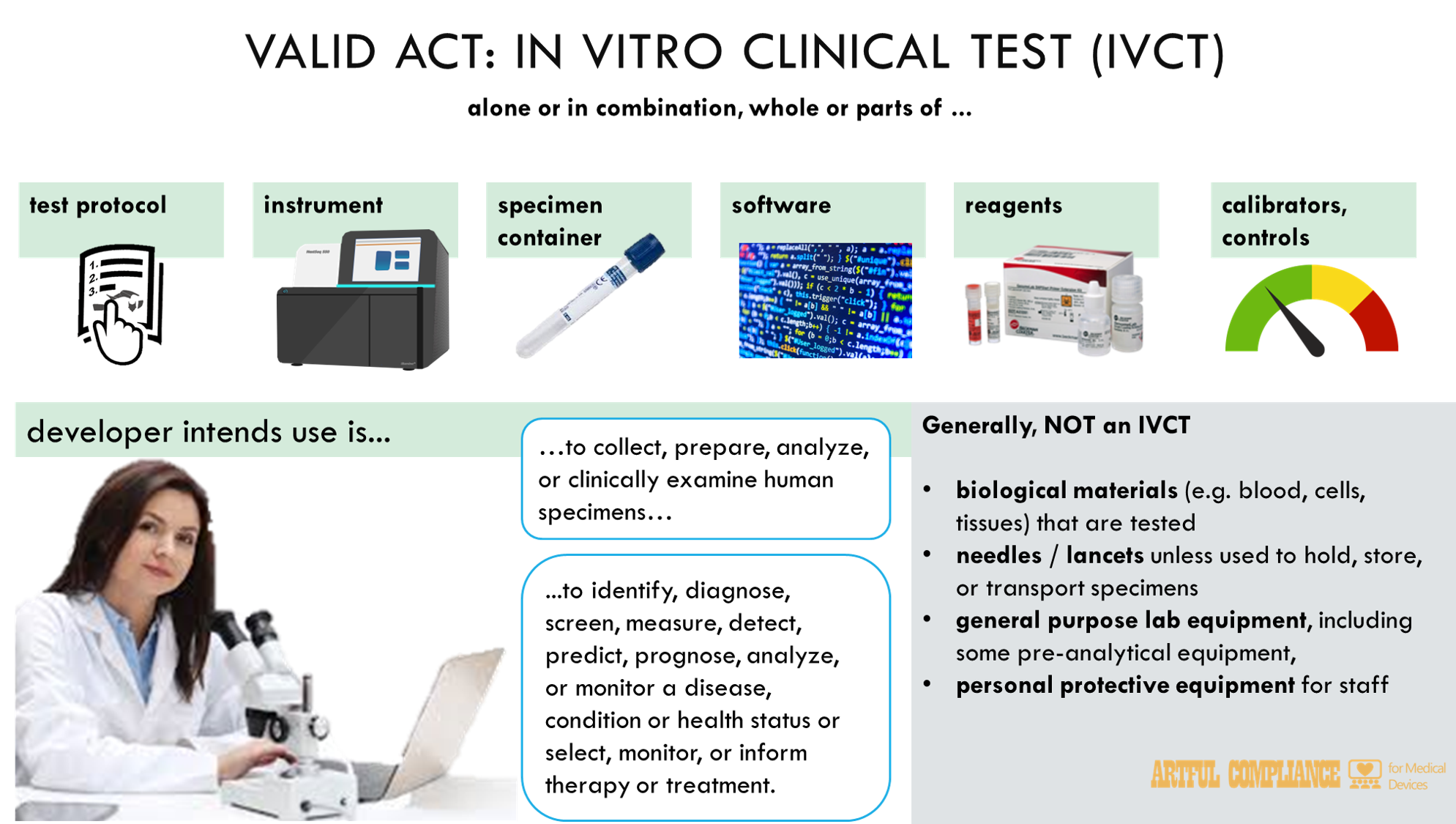
“(1) The term ‘in vitro clinical test’—
“(A) means a test intended by its developer (as defined in section 587) to be used in the collection, preparation, analysis, or in vitro clinical examination of specimens taken or derived from the human body for the purpose of—
“(i) identifying or diagnosing a disease or condition;
“(ii) providing information for diagnosing, screening, measuring, detecting, predicting, prognosing, analyzing, or monitoring a disease or condition, including by making a determination of an individual’s state of health; or
“(iii) selecting, monitoring, or informing therapy or treatment for a disease or condition; and
“(B) may include—
“(i) a test protocol or laboratory test protocol;
“(ii) an instrument (as defined in section 587(11));
“(iii) a specimen receptacle;
“(iv) software, excluding software that is excluded by section 520(o) from the definition of a device under section 201(h), and excluding modifications that are exempt in accordance with section 587A(l)(2)(A); and
“(v) subject to subparagraph (2), a component or part of a test, a test protocol, an instrument, an article, or software described in any of clauses (A) through (D) of such subparagraph, whether alone or in combination, including reagents, calibrators, and controls.
“(2) Notwithstanding subparagraph (1)(v), an article intended to be used as a component or part of an in vitro clinical test described in subparagraph (1) is excluded from the definition in subparagraph (1) if the article consists of any of the following:
“(A) Blood, blood components, or human cells or tissues, from that collected such article.
“(B) An article used for invasive sampling, a needle, or a lancthe time of acquisition, donation, or recovery of such article, including determination of donor eligibility, as applicable, until such time as the article is released as a component or part of an in vitro clinical test by the establishment et, except to the extent such article, needle, or lancet is an integral component of an article for holding, storing, or transporting a specimen.
“(C) General purpose laboratory equipment, including certain pre-analytical equipment, as determined by the Secretary.
“(D) An article used solely for personal protection during the administering, conducting, or otherwise performing of test activities.”;
(2) by adding at the end of section 201(g) the following:
“(3) The term ‘drug’ does not include an in vitro clinical test.”; and
(3) in section 201(h), by striking “section 520(o)” and inserting “section 520(o) or an in vitro clinical test”.
(b) Exclusion from definition of biological product.—Section 351(i)(1) of the Public Health Service Act (42 U.S.C. 262(i)(1)) is amended—
(1) by striking “(1) The term ‘biological product’ means” and inserting “(1)(A) The term ‘biological product’ means”; and
(2) by adding at the end the following:
“(B) The term ‘biological product’ does not include an in vitro clinical test as defined in section 201(ss) of the Federal Food, Drug, and Cosmetic Act.”.
(c) In vitro clinical test definition.—In this Act, the term “in vitro clinical test” has the meaning given such term in section 201(ss) of the Federal Food, Drug, and Cosmetic Act, as added by subsection (a).
“(18) TEST.—The term ‘test’, unless otherwise provided, means an in vitro clinical test.
Recent Comments