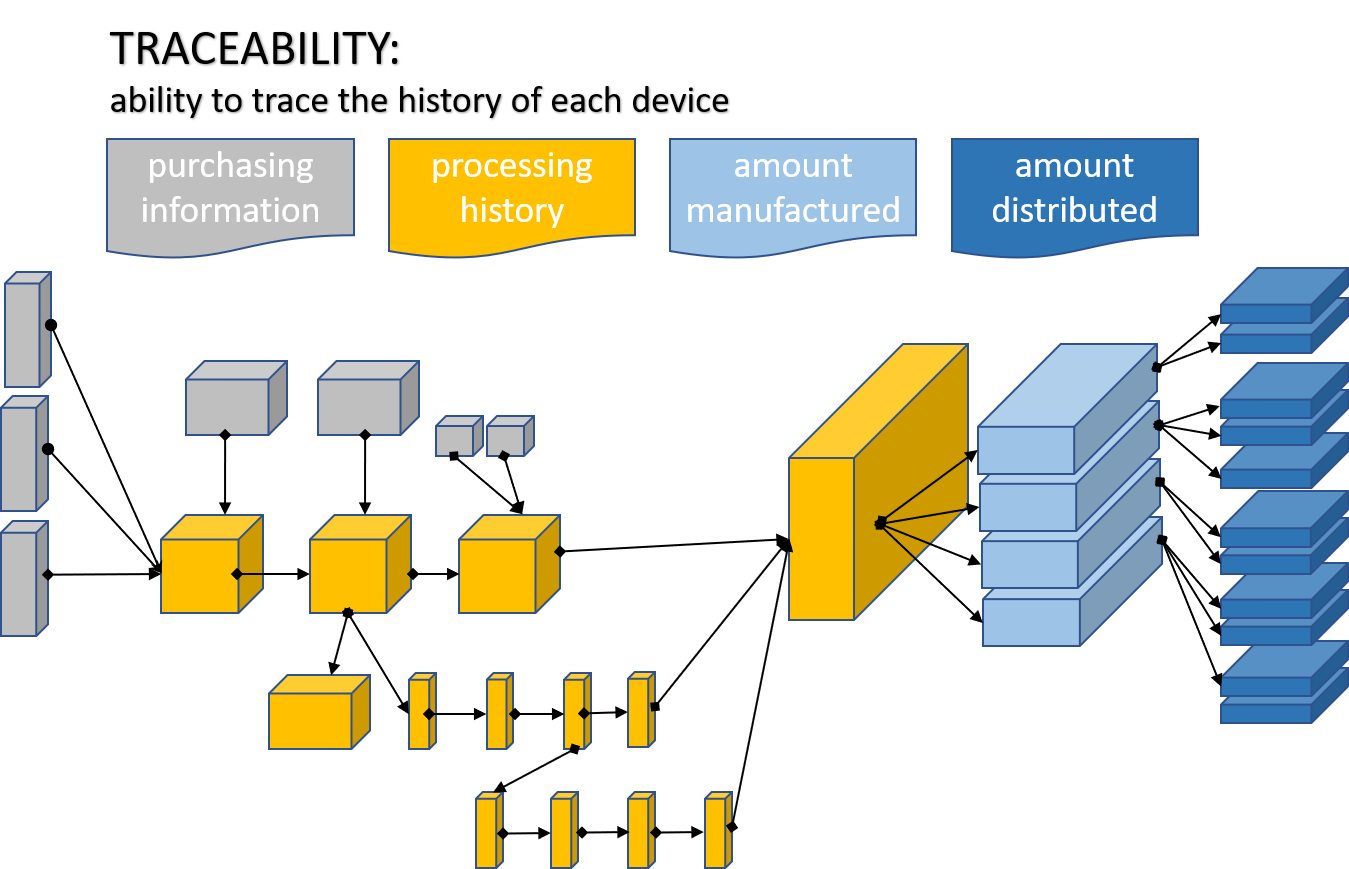
Per ISO 9000:2015, section 3.6.13, traceability is the “ability to trace the history, application or location of an object.” When considering a product or a service, traceability can relate to:
- the origin of materials and parts
- the processing history
- the distribution and location of the product or service after delivery.
Per ISO 13485:2106 section 7.5.9.1, the organization must “document procedures for traceability. These procedures shall define the extent of traceability in accordance with applicable regulatory requirements and the records to be maintained.”
What records need to be maintained?
- Relevant purchasing information in the form of documents and records” (ISO 13485:2106 section 7.4.2)
- A record of traceability for “each medical device or batch of medical devices” that “identifies the amount manufactured and amount approved for distribution” The record shall be verified and approved. (ISO 13485:2106 section 7.5.1)
Recent Comments