This article provides an overview of the key parts of 21 CFR Part 820, also called the Quality System Regulation, or just QSR, so called because it tells medical device manufacturers, importers, and distributors how to establish a quality system.
Key Terms before we go on:
- The word ‘establish’ is used 80 times in the regulation, and means to define, document, and implement.
- A quality system means the organizational structure, responsibilities, procedures, processes, and resources to ensure the safety and effectiveness of medical devices.
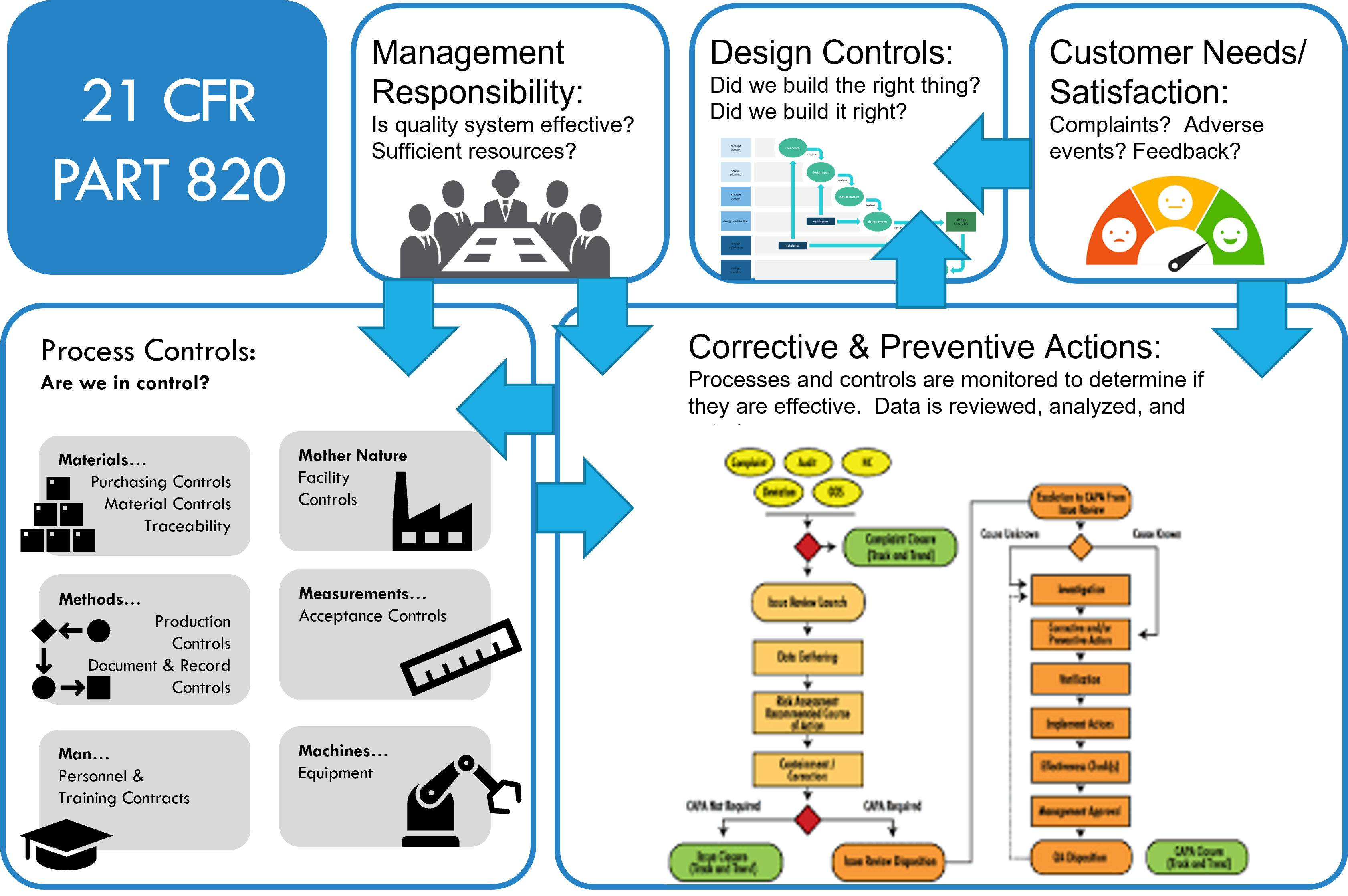
Management Responsibility
Management must establish a quality policy, assign quality roles and responsibilities, provide adequate resources, conduct management reviews, and ensure effective communication within the organization and with customers and regulators.
Design Controls
Establish a way to control the design and development of medical devices, from the initial planning to the final transfer to production. Define design inputs (what a device needs to do) and outputs (how you make sure it does that), conduct design reviews and verification and validation activities, manage design changes, and maintain design history records.
Customer Needs / Satisfaction
Identify and meet the needs and expectations of its customers, including the end users of the medical devices.
Establish a way to determine customer requirements, communicate with customers, handle customer complaints, and measure customer satisfaction.
Process Controls
Establish a way to control the processes that affect the quality of the medical devices, from the receipt of raw materials to the delivery of finished products. The process controls include the following:
Materials
Ensure the materials that end up in the medical devices are suitable, safe, and conform to specifications. Control the purchase, receipt, inspection, storage, handling, and distribution of those materials.
Mother Nature
Ensure that manufacturing environmental conditions, such as temperature, humidity, lighting, and cleanliness, are appropriate for the medical device processes and do not negatively affect the quality of the products.
Methods
Ensure that the methods used in the medical device processes are documented, validated, and followed. The methods include the procedures, instructions, standards, and techniques for performing the processes.
Measurements
Ensure the measurements used in the medical device processes are accurate, reliable, and traceable. The measurements include the inspection, testing, calibration, and monitoring of the processes and products.
Man
Ensure the personnel involved in the medical device making processes are qualified, trained, and competent. Provide a safe and healthy work environment for personnel.
Machines
Ensure the equipment and software used in the medical device processes are suitable, maintained, and controlled. The equipment and software include the tools, instruments, devices, and programs that perform the manufacturing and testing processes.
Corrective and Preventive Actions
Establish a way to identify, analyze, correct, and prevent the causes of nonconformities (failures to meet requirements) in the medical device processes and products. Investigating the root causes of nonconformities, implement corrective and preventive measures, verify the effectiveness of actions taken, and document the results.
Recent Comments