The FDA needs to agree a medical device is reasonably safe and effective before you can sell it in the US. For Class II (moderate risk devices), this agreement is called ‘clearance’. To get ‘clearance’, you’ll need to submit evidence your device is safe and effective (a process called a ‘premarket notification’). In this article, we will explain what a 510(k) submission is, when it is required, how to prepare it, and what to expect from the FDA review process.
What is a 510(k) submission?
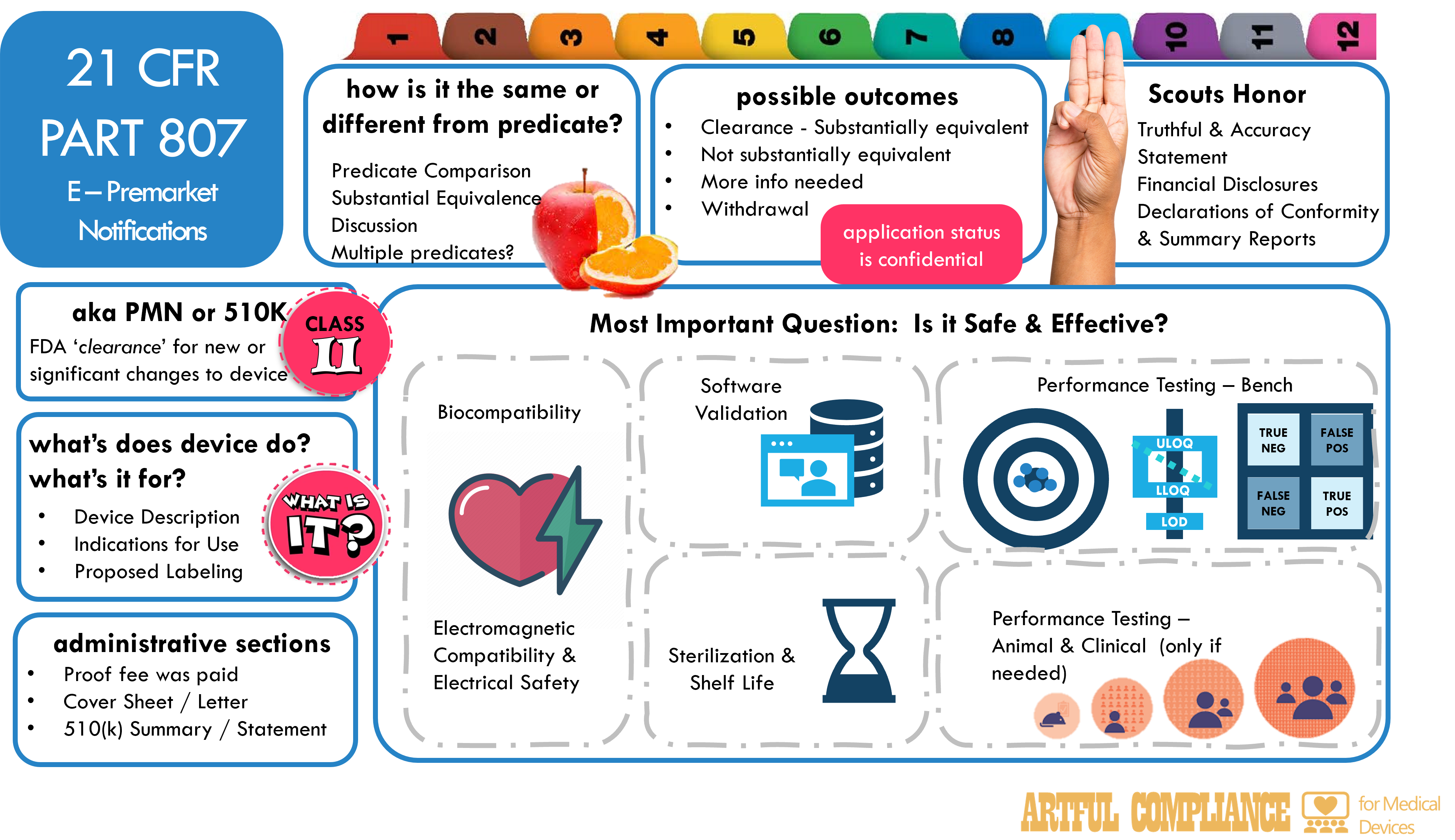
A 510(k) submission is a document that shows the FDA your device is safe and effective for its intended use, and that it is substantially equivalent to a legally marketed device (also known as a predicate device). In case you were wondering, its known as a 510(k) submission, because the regulation is based on section 510K of the Federal Food, Drug, and Cosmetic Act (FD&C). Substantial equivalence means that your device has the same intended use, technological characteristics, and performance as the predicate device, or that any differences do not raise new questions of safety and effectiveness.
When is a 510(k) submission required?
You need to submit a 510(k) to the FDA if:- Your device is new or not substantially equivalent to an existing device (this process is called a ‘de novo’ and it allows the FDA to create a new category of device). You selling a device and the registered manufacturer, importer, or repackager has changed.
- You significantly change or modify the devices design, components, manufacture, or intended use.
- Your device is exempt by regulation or order (see FDA’s list of exempt devices)
- Your device is for export only
- Your device is for investigational use only (see 21 CFR Part 812).
What information is required in a 510(k) submission?
A 510(k) submission must include the following information:What Does Device Do? What is it for?
- Device description: A description that provides the name, the classification, the product code, the regulatory status, the marketing history, and the physical and technical characteristics of the device
- Indications for use statement: A statement that describes the intended use of the device, including the patient population, the disease or condition, and the mode of application
- Proposed labeling: A copy of the proposed labeling for the device, including the instructions for use, the indications, the contraindications, the warnings, the precautions, and the adverse effects
Comparison to Predicate(s)
- Substantial equivalence discussion: A discussion that compares the device with the predicate device(s) already on the market in terms of intended use, technological characteristics, and performance, and explains how any differences do not raise new questions of safety and effectiveness.
Answer Most Important Question: Is it Safe and Effective?
- Performance testing: A description of the performance testing (bench, animal, and/or clinical) and results for the device, to demonstrate its safety and effectiveness for its intended use.
- Animal and clinical testing: If applicable, a description of any animal and clinical testing and results for the device, if applicable, including the study design, the study population, the study endpoints, the statistical analysis, and the ethical considerations. NOTE: Class II devices generally do not require animal or clinical testing.
- Sterilization and shelf life: A description of the sterilization method, the sterility assurance level, the packaging, the storage conditions, and the shelf life of the device, if applicable
- Biocompatibility: A description of the biocompatibility testing and results for the device, if it contacts human tissues or fluids
- Software: A description of the software design, development, verification, validation, and hazard analysis for the device, if it contains software
- Electromagnetic compatibility and electrical safety: A description of the electromagnetic compatibility and electrical safety testing and results for the device, if it emits or is affected by electromagnetic radiation.
- Additional information: Any other information that may be relevant or requested by the FDA, such as risk analysis, literature review, reference standards, etc.
Honesty Stuff
- Truthful and accuracy statement: A statement that certifies that all information in the submission is truthful and accurate, and that no material fact has been omitted
- Financial certification or disclosure statement: A certification or a disclosure that are required for clinical investigators who conducted or participated in clinical studies that support the 510(k) (see 21 CFR Part 54)
Administrative Stuff
- User fee cover sheet: A form that identifies the submitter and the type of submission, and confirms the payment of the FDA’s fee.
- 510(k) cover letter: A letter that summarizes the purpose and content of the submission, and provides the contact information of the official correspondent.
- 510(k) summary or statement: A summary that provides an overview of the device, the predicate device, and the substantial equivalence comparison, or a statement that agrees to make the complete 510(k) available upon request.
How to format a 510(k) submission?
A 510(k) submission are now required to be electronic and submitted using the FDA’s [Electronic Submissions Gateway (ESG)] and eCopy Program. A 510(k) submission can also be formatted in different ways depending on the type and number of devices. For example:- Multiple devices: A 510(k) can include more than one device, if they have the same intended use, the same technological characteristics, and the same performance specifications. The submission should clearly identify and distinguish each device and its corresponding information.
- Abbreviated 510(k): A 510(k) can follow an abbreviated format, if it relies on the use of FDA-recognized standards to demonstrate substantial equivalence. The submission should include a summary report that describes how the device conforms to the standards or guidance.
- Special 510(k): A 510(k) submission can follow a special format, if it is submitted by the manufacturer of a legally marketed device, and it requests a modification to the device’s design, labeling, or indications. The submission should include a declaration of conformity to design control requirements.
What is a 510(k) summary?
A 510(k) summary sometimes called a ‘technical summary’, is a document that provides an overview of the device, the predicate device, and the substantial equivalence comparison. It is intended to be a concise and accurate summary of the 510(k) submission, and it is made available to the public by the FDA. A 510(k) summary must include the following information:- Applicant’s name and address: The name and address of the person or entity who submitted the 510(k)
- Correspondent’s name and address: The name and address of the person who is authorized to communicate with the FDA about the 510(k)
- Device name: The trade or proprietary name, the common or usual name, and the classification name and product code of the device
- Predicate device name and 510(k) number: The name and the 510(k) number of the legally marketed device that is claimed as the predicate device
- Device description: A brief description of the device, including its function, principle of operation, and technological characteristics
- Intended use: A statement of the intended use of the device, including the patient population, the disease or condition, and the mode of application
- Summary of technological characteristics: A summary of the technological characteristics of the device and the predicate device, and a comparison of their similarities and differences
- Summary of performance data: A summary of the performance data (bench, animal, and/or clinical) that support the safety and effectiveness of the device for its intended use
- Conclusions: A statement of the conclusions drawn from the comparison of the device and the predicate device, and the demonstration of substantial equivalence
What is a 510(k) statement?
A 510(k) statement is a document that agrees to make the complete 510(k) submission available to any person within 30 days of a written request. It is an alternative to a 510(k) summary, and it is not made available to the public by the FDA. A 510(k) statement must include the following information:- Statement that all information in the submission is truthful and accurate, and that no material fact has been omitted
- Statement that the submitter will make the complete 510(k) available to any person within 30 days of request and specifies the address where such requests should be sent
- Name, title, address, telephone number, and signature of the official correspondent with the FDA
What to expect from the FDA review process
- pre-submissions: These are technically optional, but before you submit pre-market notification, you should have at least one pre-submission with the FDA to get feedback on the key aspects of your submission. It will save you time and hassle. I’ll cover pre-submissions in a later article.
- online Registration process on CDRH Portal assigns a number to each 510 (k) submission. There is a monitoring tool and contact information for the FDA lead reviewer. The fact that you are submitting is confidential.
- acceptance review: The FDA will check if the submission is complete, the fee is paid, and meets the minimum requirements for review. This usually takes 15 days once received. The FDA will issue an acceptance or a refusal letter to the submitter.
- substantive review: The FDA will conduct a detailed review of the submission to determine if the device is substantially equivalent to the predicate device, safe, and effective. This usually takes 60 days. Communication is typically either:
- an email stating that FDA will proceed to resolve any outstanding deficiencies using Interactive Review (back and forth emails); or
- an Additional Information (AI) request which places the submission on hold for up to 180 days.
- Note that the FDA has a goal of 90 FDA days to issue a decision letter with the findings of substantial equivalence or not, but this does not could the time waiting for the submitter to respond.
- marketing clearance: If the FDA finds the device substantially equivalent, the submitter can market the device in the U.S. subject to the general and special controls applicable to the device. The FDA will list the device on its 510(k) database and publish the 510(k) summary or statement on its website.
Recent Comments