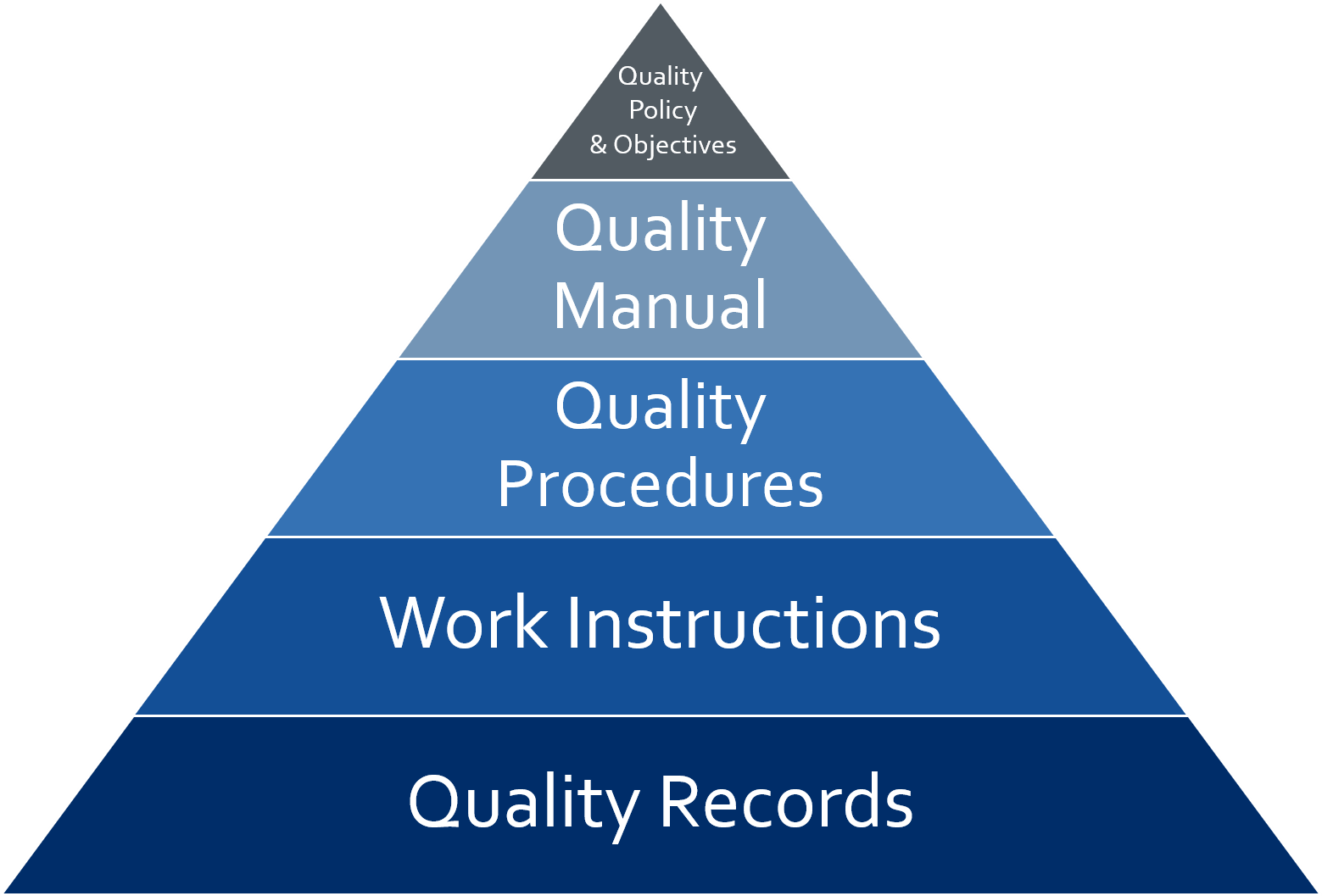
Per ISO 13485:2016, Section 4.2.1, General: “The quality management system documentation shall include: a) documented statements of a quality policy and quality objectives; b) a quality manual; c) documented procedures and records required by this International Standard; d) documents, including records, determined by the organization to be necessary to ensure the effective planning, operation, and control of its processes; e) other documentation specified by applicable regulatory requirements
PowerPoint: PPT Quality Document Hierarchy
Recent Comments