The FDA has published a proposed rule to amend 21 CFR Part 809 (in vitro diagnostic) regulations to state that laboratory developed tests (LDTs) are in vitro diagnostic products (IVDs) designed, manufactured and used within a single clinical laboratory, and need to follow the same rules as other medical devices.
What you should know:
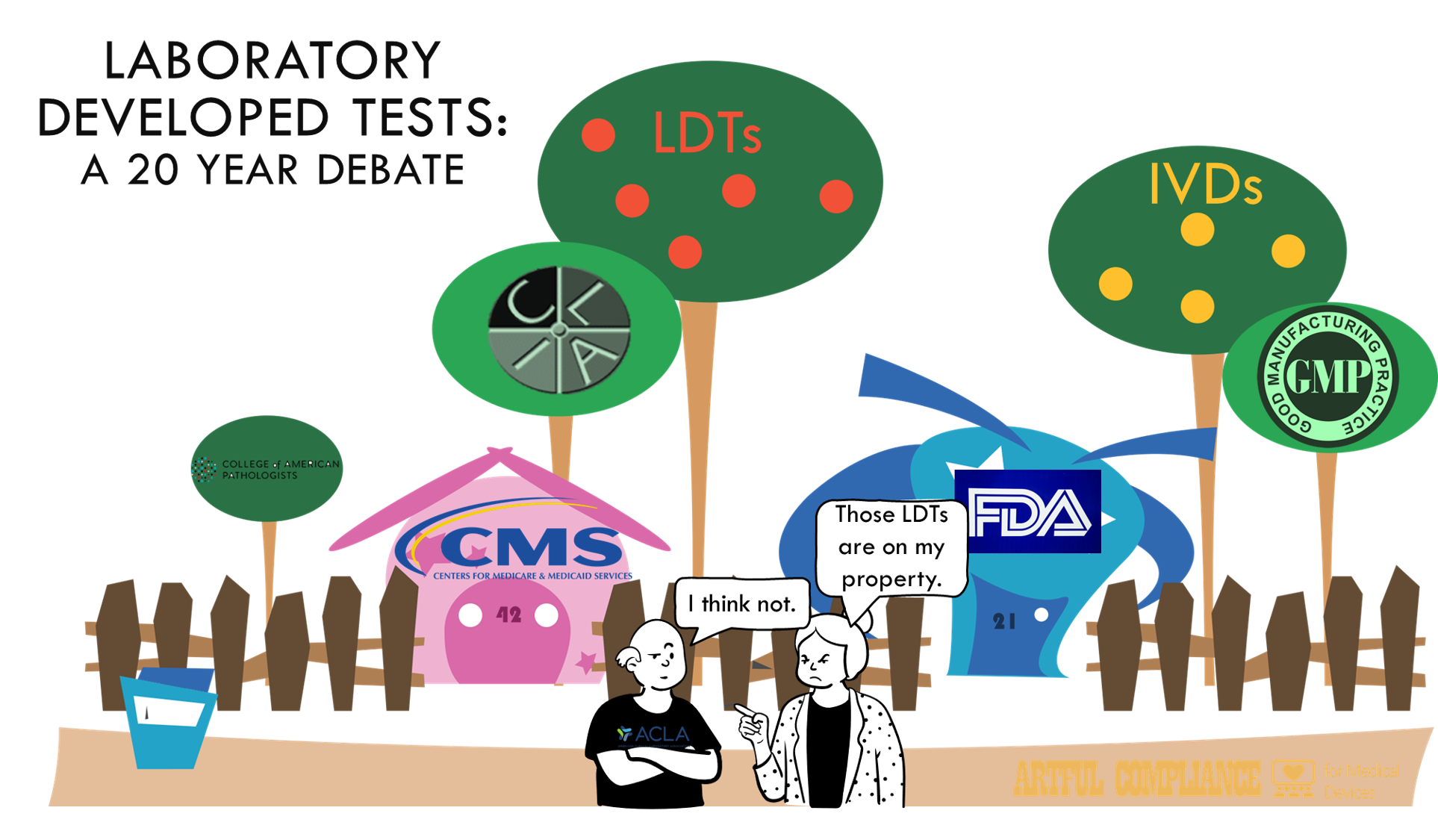
- The FDA believes LDTs are IVDs and it’s going to regulate them as such. This is a point that should come as no surprise as the FDA has made it ad nauseum for going on 20 years now. The FDA’s oft-repeated line is that in the almost 50 years since the 1976 Medical Device Amendments, it has simply been practicing ‘enforcement discretion’. In other word’s it has simply decided not to do anything about the thousands of labs not following the IVD regulations, but always maintaining they could if they felt it was needed.
- The FDA has a long (unsuccessful) history of trying to start enforcing IVD rules for LDTs. It started 20+ years ago. Jeffery Gibbs provides a comprehensive review of this in LDTs: The Saga Continues. The whole thing reads like two neighbors arguing over a property line. Industry, and especially academic medical centers have fought the enforcement tooth and nail. The latest failure to get the VALID act through Congress appears to have been the FDA’s last straw.
- The FDA expects a legal battle. Using the ‘rule-making process’, to call LDTs as IVDs effectively bypasses Congress and goes straight into the process to simply update the regulation (21 CFR Part 809). It’s the equivalent of saying the property line is ten feet over and simply starting the process to move the fence. Since the VALID act went to Congress in the first place, that would seem to imply the FDA knows Congressional action was needed to change the regulation. The challenge of FDA’s legal authority is bound to be a great show. Bring the popcorn.
- Exemptions and Exceptions. The rule provides a smorgasbord of labs and tests that may be exempt (e.g. blood typing, forensics), might have less stringent rules (academic labs, low revenue labs, tests approved by New York or the VHA), or that might get additional transition time. It asks for feedback from the public on all of these possibilities, in what will almost certainly be a very long comment review process.
- Benefits & Costs. Per the FDA, the rule is aimed at helping to ensure the safety and effectiveness of LDTs and improving innovation. Most of the 83 page document in the Federal Register are devoted to why the FDA believes LDTs are unsafe. Sure, improving patient safety is great. But labs are nervously eyeing the price tag. The FDA outlines benefits ($86B/year) and costs of the rule (5.8B to labs, plus 500M to FDA that will be passed off to labs as user fees). It’s anyone’s guess what magic hat the FDA pulled those estimates from. The Pew Trust researched the topic in 2021 and concluded it was ‘difficult to know precisely how many LDTs are on the market, or to accurately estimate the volume of tests that are run using LDTs [but] they are clearly common, and many labs rely on them in some capacity.”
- Timeline. As you might expect, the FDA’s plan is to phase in the new approach. See graphic below. Year one starts with adverse event reporting and the ability to recall IVDs. Year two will require registration and listing. Year three will bring QMS requirements (design and manufacturing controls, CAPAs, complaints, etc under 21 CFR Part 820) which by that time might actually be updated to align with ISO 13485. Lastly, year 3.5 and 4 will mark deadlines for FDA application for Class III PMA and Class I/II de novos or 510Ks.
- What Does the Future Hold? Considering the concerns that the FDA can’t even handle its current workload with IVDs, no doubt these cut-offs will be followed with a lengthy period of years in which the absolutely overloaded FDA will wade through a backfill funded by astronomical user fees that will still not cover the work that needs to be done as inexperienced labs take on their first FDA applications. If you are a lab with one or more LDTs, the future holds much uncertainty. If you are a regulatory affairs professional in the IVD industry, expect job security and a raise.

Recent Comments