00090 VALID Act: Conditions of Approval and Approval Publication
From VALID Act “SEC. 587B. Premarket review.
“(2) APPROVAL OF AN APPLICATION.—
….
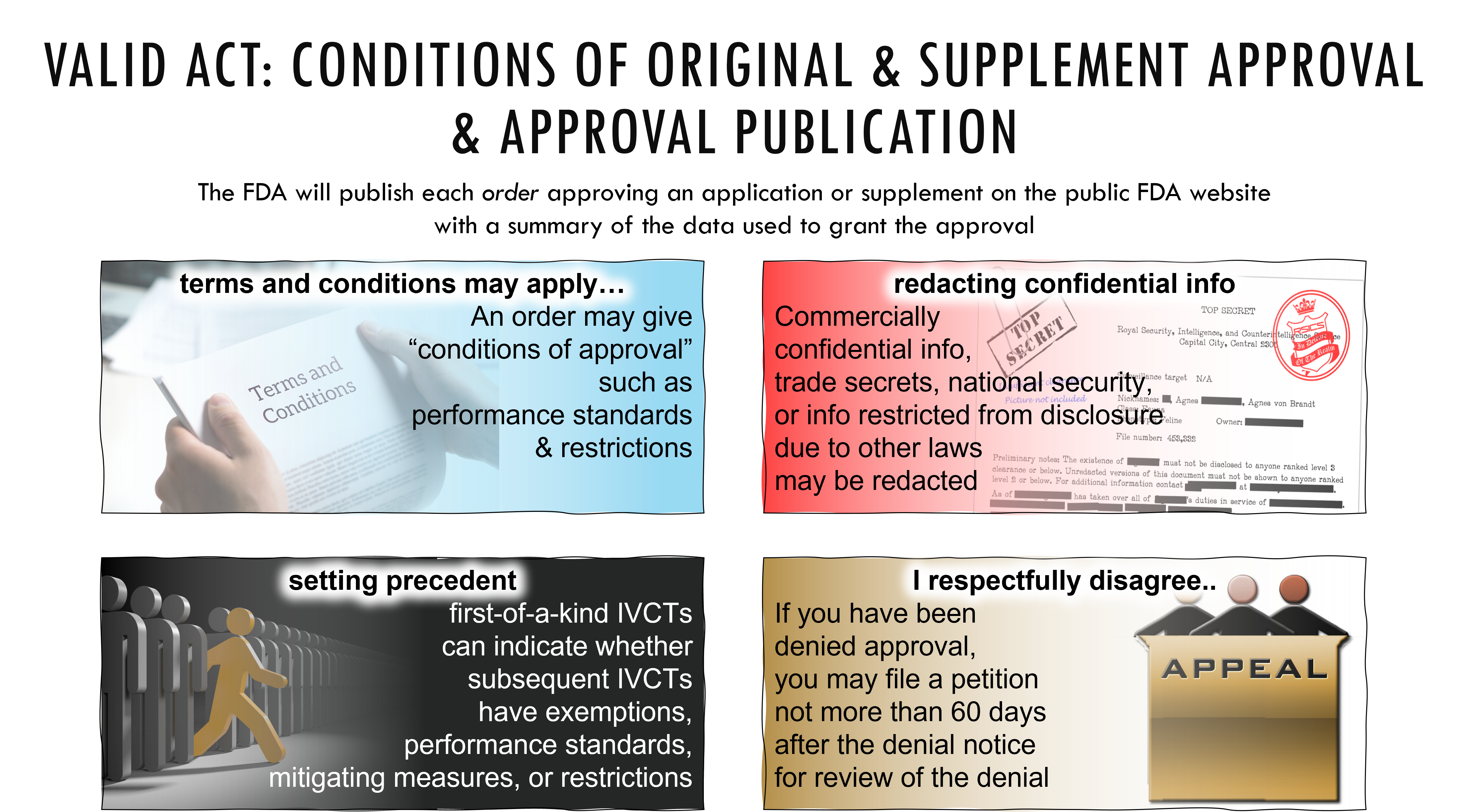
“(B) CONDITIONS OF APPROVAL.—An order approving an application pursuant to this paragraph may require conditions of approval for the in vitro clinical test, including conformance with performance standards under section 587Q and restrictions under section 587N.
“(C) FIRST-OF-A-KIND TEST.—For a first-of-a-kind in vitro clinical test, an order approving an application pursuant to this paragraph—
“(i) may impose requirements for tests with the same indications for use, including conformance with performance standards under section 587Q and mitigating measures under section 587E, and comply with restrictions under section 587N; and
“(ii) shall indicate whether subsequent in vitro clinical tests with the same intended use may meet an exemption set forth in section 587A.
“(D) PUBLICATION.—The Secretary shall publish each order approving an application pursuant to this paragraph on the public website of the Food and Drug Administration and make publicly available a summary of the data used to grant the approval, except to the extent the Secretary determines that such order—
“(i) contains commercially confidential or trade secret information; or
“(ii) relates to national security or countermeasures is restricted from disclosure pursuant to statutory provisions other than this section.
“(3) REVIEW OF DENIALS.—An applicant whose application submitted under subsection (c) or (d) has been denied approval may, by petition filed not more than 60 calendar days after the date on which the applicant receives notice of such denial, obtain review of the denial in accordance with section 587O.
From VALID Act ““SEC. 587B. Premarket review.
“(5) CONDITIONS OF APPROVAL.—In an order approving a supplement under this subsection, the Secretary may require conditions of approval for the in vitro clinical test, including compliance with restrictions under section 587N and conformance to performance standards under section 587Q.
“(6) APPROVAL.—The Secretary shall approve a supplement under this subsection if—
“(A) the data demonstrate that the modified in vitro clinical test meets the applicable standard; and
“(B) the holder of the application approved under subsection (g) for the test has demonstrated compliance with applicable quality and inspection requirements, as applicable and appropriate.
“(7) PUBLICATION.—The Secretary shall publish on the public website of the Food and Drug Administration notice of any order approving a supplement under this subsection, except that such publication shall exclude—
“(A) commercial confidential or trade secret information; and
“(B) any other information that the Secretary determines to relate to national security or countermeasures or to be restricted from disclosure pursuant to another provision of law.
“(8) REVIEW OF DENIAL.—An applicant whose supplement under this subsection has been denied approval may, by petition filed on or before the 60th calendar day after the date upon which the applicant receives notice of such denial, obtain review of the denial in accordance with section 587O.
Recent Comments