00061 Grandfathered Tests
“(c) Grandfathered tests.—
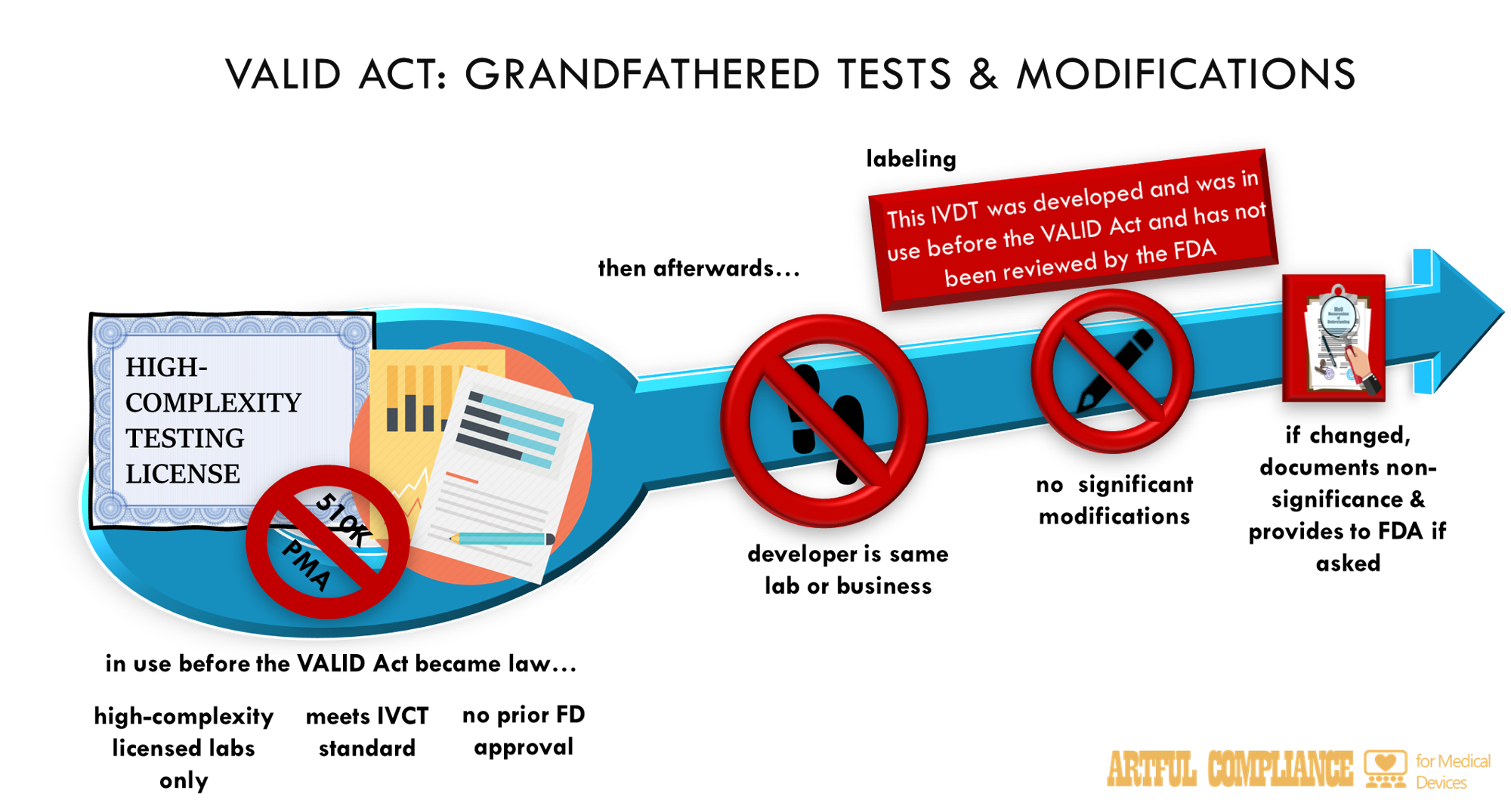
“(1) EXEMPTION.—An in vitro clinical test that meets the criteria set forth in paragraph (2) is exempt from the requirements of this subchapter, except as provided under subsection (a)(4), the registration and listing requirements under section 587I, and the adverse reporting requirements under section 587L, and may be lawfully marketed subject to the other applicable requirements of this Act, if—
“(A) each test report template for the test bears a statement of adequate prominence that reads as follows: “This in vitro clinical test was developed and first introduced prior to the date of enactment of the Verifying Accurate Leading-edge IVCT Development Act of 2021 and has not been reviewed by the Food and Drug Administration.”; and
“(B) the developer of the test—
“(i) maintains documentation demonstrating that the test meets and continues to meet the criteria set forth in paragraph (2); and
“(ii) makes such documentation available to the Secretary upon request.
“(2) CRITERIA FOR EXEMPTION.—An in vitro clinical test is exempt as specified in paragraph (1) if the test—
“(A) (i) was first offered for clinical use by such laboratory before the date of enactment of the Verifying Accurate Leading-edge IVCT Development Act of 2021;
“(ii) was developed by a clinical laboratory for which a certificate was in effect under section 353 of the Public Health Service Act that meets the requirements under such section 353 for performing high-complexity testing; and
“(iii) is performed—
“(I) in the same clinical laboratory in which it was developed;
“(II) by another clinical laboratory for which a certificate is in effect under section 353 within the same corporate organization and having common ownership by the same parent corporation; or
“(III) by a laboratory within a public health laboratory network coordinated or managed by the Centers for Disease Control and Prevention;
“(B) does not have in effect an approval under section 515, a clearance under section 510(k), an authorization under section 513(f)(2), or an exemption under section 520(m); and
“(C) is not modified on or after the date of enactment of the Verifying Accurate Leading-edge IVCT Development Act of 2021 by its initial developer (or another person) in a manner such that the test is a new in vitro clinical test under subsection (l).
“(3) MODIFICATIONS.—In the case of a modification to an in vitro clinical test that is exempt as specified in paragraph (1) or such modification is otherwise not subject to premarket review pursuant to section 587A(l), the test continues to qualify for such exemption if the person modifying such test—
“(A) documents each such modification and maintains a summary of the basis for such determination; and
“(B) provides such documentation and summary to the Secretary upon request or inspection. (d) Tests exempt from section 510(k).—
“(1) EXEMPTION.—An in vitro clinical test is exempt from premarket review under section 587B and may be lawfully marketed subject to the other applicable requirements of this Act, if the in vitro clinical test—
“(A) (i) was offered for clinical use prior to the date of enactment of the Verifying Accurate Leading-edge IVCT Development Act of 2021; and
“(ii) immediately prior to such date of enactment was exempt pursuant to subsection (l) or (m)(2) of section 510 from the requirements for submission of a report under section 510(k); or
“(B) (i) was not offered for clinical use prior to such date of enactment;
“(ii) is not a test platform; and
“(iii) falls within a category of tests that was exempt from the requirements for submission of a report under section 510(k) as of such date of enactment (including class II devices and excluding class I devices described in section 510(l)).
Recent Comments