
PPT: PPT-00035 REV01 Investigational IVD Types
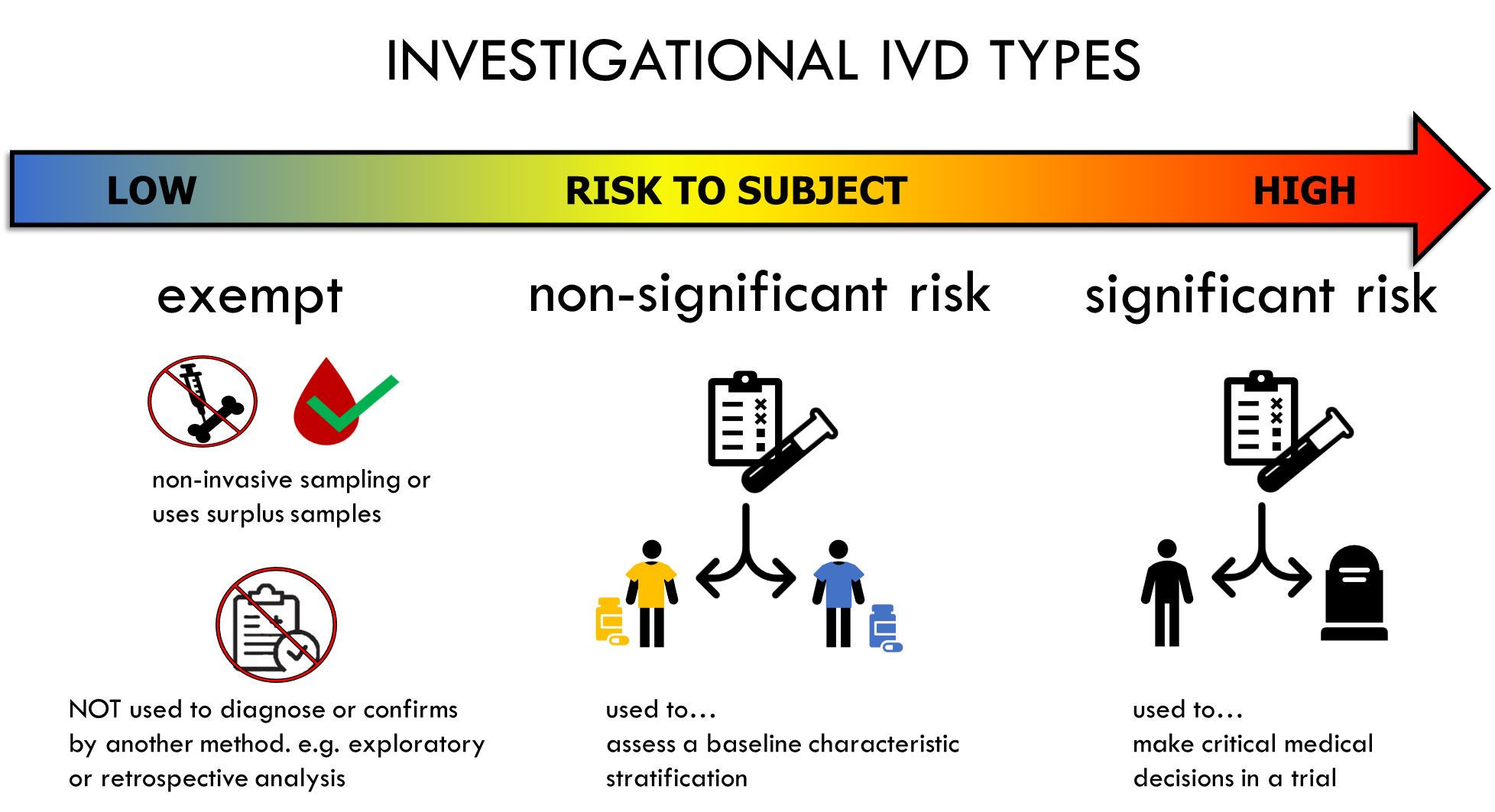
Investigational Device IVD per 21 CFR Part 812 include ‘exempt’, ‘non-significant risk, and ‘significant risk’ IVDs. The classification is based on the risk to the subject participating in the clinical trial. The category determines the applicable IDE requirements.
Recent Comments