
PPT-00034 REV01 Companion Diagnostic Definition
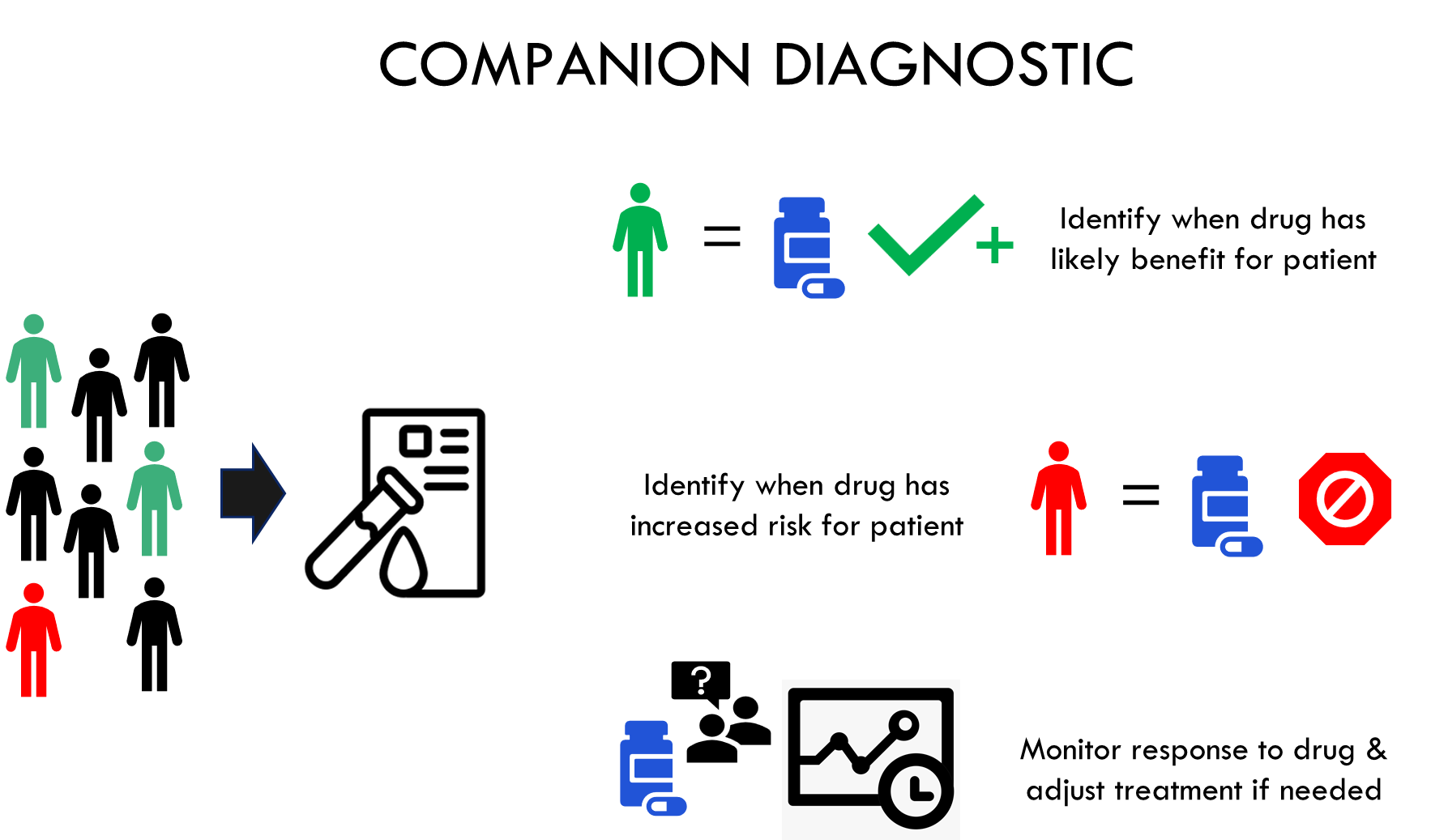
IVD companion diagnostics are, by definition, essential for the safe and effective use of a corresponding therapeutic product and may be used to:
1) identify patients who are most likely to benefit from the therapeutic product;
2) identify patients likely to be at increased risk for serious adverse reactions as a result of treatment with the therapeutic product;
3) monitor response to treatment with the therapeutic product for the purpose of adjusting treatment (e.g., schedule, dose, discontinuation) to achieve improved safety or effectiveness; or
4) identify patients in the population for whom the therapeutic product has been adequately studied and found to be safe and effective (i.e., there is insufficient information about the safety and effectiveness of the therapeutic product in any other population).
“In Vitro Companion Diagnostic Devices,” FDA Guidance
Recent Comments