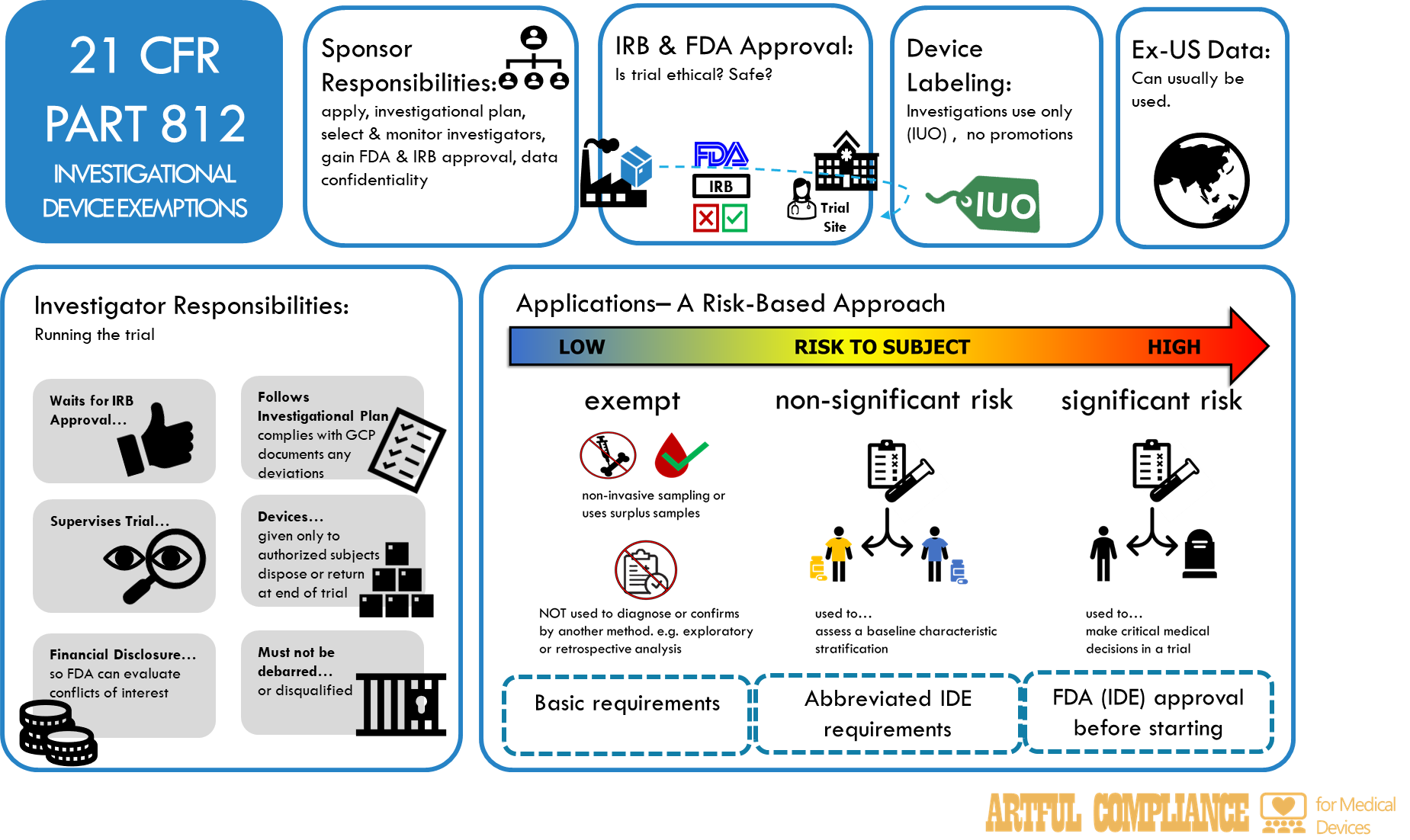
00091 REV01 21 CFR Part 812 Investigational Device Exemptions
Part 812Investigational Device Exemptions812.1 – 812.150
Subpart A General Provisions 812.1 – 812.19
- 812.1Scope.
- 812.2Applicability.
- 812.3Definitions.
- 812.5Labeling of investigational devices.
- 812.7Prohibition of promotion and other practices.
- 812.10Waivers.
- 812.18Import and export requirements.
- 812.19Address for IDE correspondence.
Subpart B Application and Administrative Action 812.20 – 812.38
- 812.20Application.
- 812.25Investigational plan.
- 812.27Report of prior investigations.
- 812.28Acceptance of data from clinical investigations conducted outside the United States.
- 812.30FDA action on applications.
- 812.35Supplemental applications.
- 812.36Treatment use of an investigational device.
- 812.38Confidentiality of data and information.
Subpart C Responsibilities of Sponsors 812.40 – 812.47
- 812.40General responsibilities of sponsors
- 812.42FDA and IRB approval.
Recent Comments