
PPT: PPT-00028 REV01 21 CFR Part 820
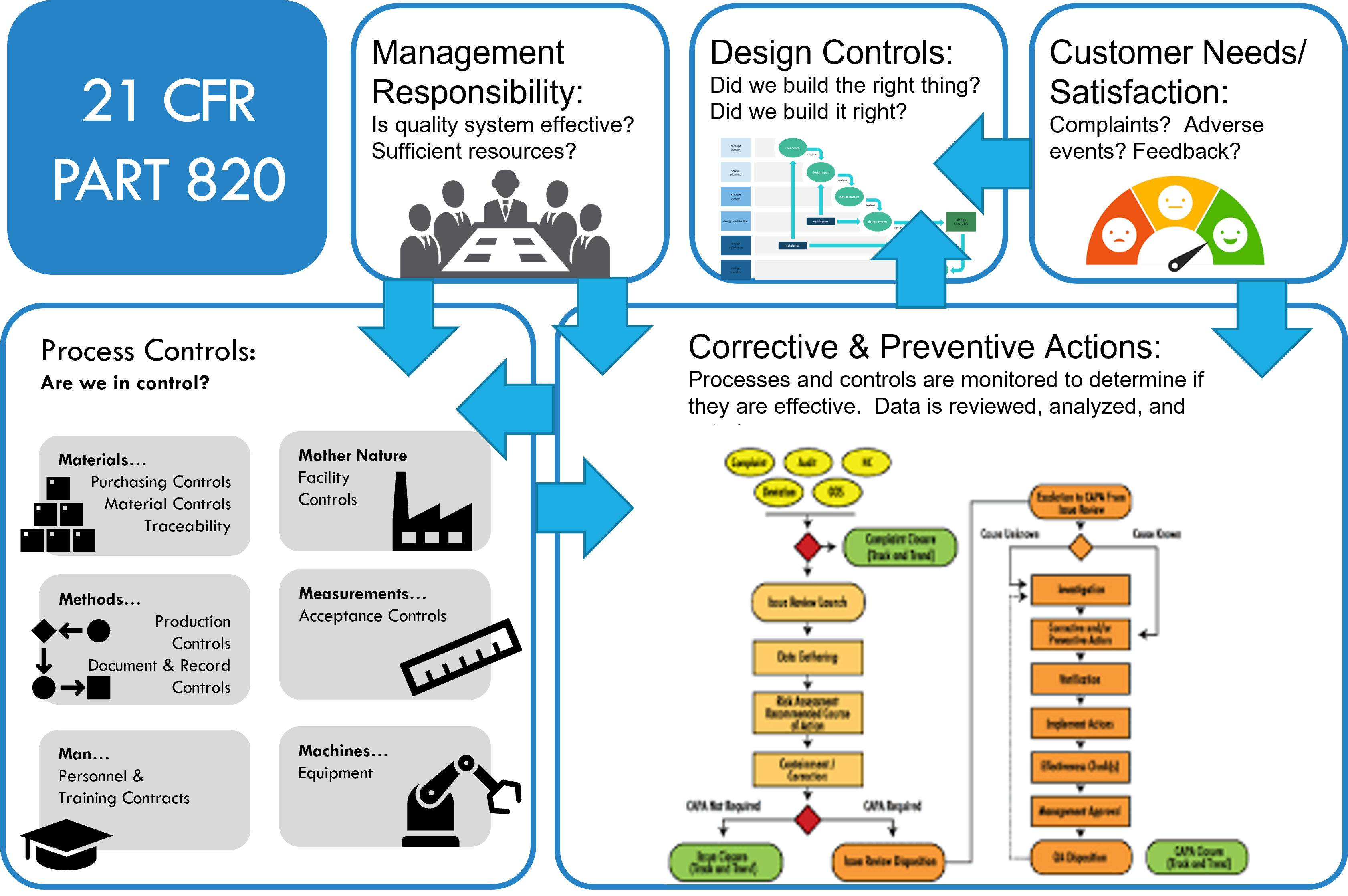
21 CFR Part 820, also known as the Quality System Regulation (QSR), is a document that outlines Current Good Manufacturing Practice (CGMP) regulations. This document governs manufactures to help ensure their products consistently meet applicable requirements and specifications. FDA 21 CFR Part 820 is the quality system approved by the FDA. These requirements are to ensure that medical devices are both safe and effective. Medical device manufacturers undergo FDA inspections to ensure FDA 21 CFR 820 compliance.
Recent Comments