IVDR ANNEX VIII CLASSIFICATION RULES
PPT: PPT-00014 REV01 IVDR IVD Classification
- IMPLEMENTING RULES
1.1. Application of the classification rules shall be governed by the intended purpose of the devices.
1.2. If the device in question is intended to be used in combination with another device, the classification rules shall apply separately to each of the devices.
1.3. Accessories for an in vitro diagnostic medical device shall be classified in their own right separately from the device with which they are used.
1.4. Software, which drives a device or influences the use of a device, shall fall within the same class as the device.
If the software is independent of any other device, it shall be classified in its own right.
1.5. Calibrators intended to be used with a device shall be classified in the same class as the device.
1.6. Control materials with quantitative or qualitative assigned values intended for one specific analyte or multiple analytes shall be classified in the same class as the device.
1.7. The manufacturer shall take into consideration all classification and implementation rules in order to establish the proper classification for the device.
1.8. Where a manufacturer states multiple intended purposes for a device, and as a result the device falls into more than one class, it shall be classified in the higher class.
1.9. If several classification rules apply to the same device, the rule resulting in the higher classification shall apply.
1.10. Each of the classification rules shall apply to first line assays, confirmatory assays and supplemental assays.
- CLASSIFICATION RULES
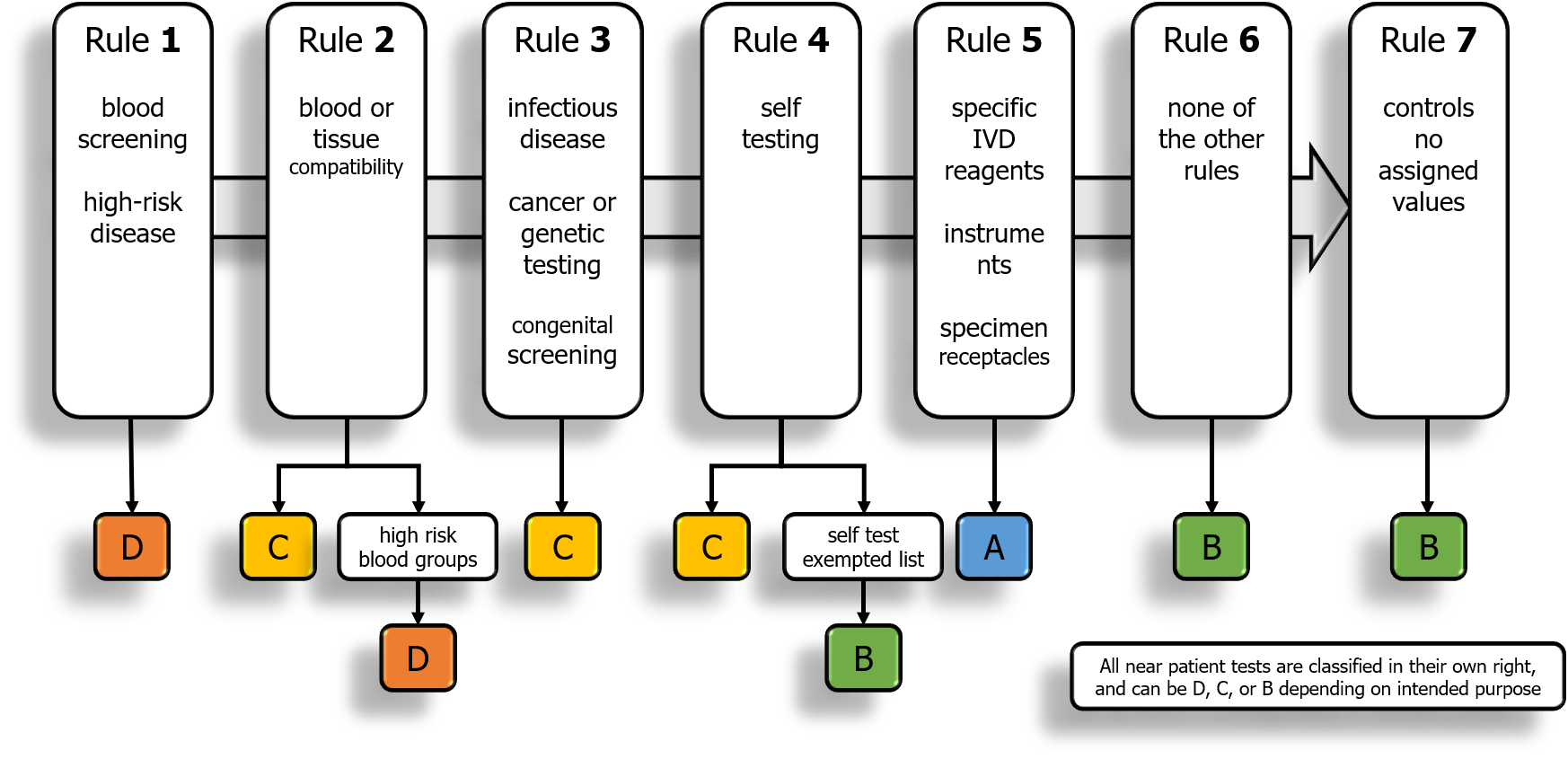
2.1. Rule 1
Devices intended to be used for the following purposes are classified as class D:
— detection of the presence of, or exposure to, a transmissible agent in blood, blood components, cells, tissues or organs, or in any of their derivatives, in order to assess their suitability for transfusion, transplantation or cell administration;
— detection of the presence of, or exposure to, a transmissible agent that causes a life-threatening disease with a high or suspected high risk of propagation;
—determining the infectious load of a life-threatening disease where monitoring is critical in the process of patient management.
2.2. Rule 2
Devices intended to be used for blood grouping, or tissue typing to ensure the immunological compatibility of blood, blood components, cells, tissue or organs that are intended for transfusion or transplantation or cell administration, are classified as class C, except when intended to determine any of the following markers:
—ABO system [A (ABO1), B (ABO2), AB (ABO3)];
— Rhesus system [RH1 (D), RHW1, RH2 (C), RH3 (E), RH4 (c), RH5 (e)];
—Kell system [Kel1 (K)];
—Kidd system [JK1 (Jka), JK2 (Jkb)];
—Duffy system [FY1 (Fya), FY2 (Fyb)];
in which case they are classified as class D.
2.3. Rule 3
Devices are classified as class C if they are intended:
(a) for detecting the presence of, or exposure to, a sexually transmitted agent;
(b) for detecting the presence in cerebrospinal fluid or blood of an infectious agent without a high or suspected high risk of propagation;
(c) for detecting the presence of an infectious agent, if there is a significant risk that an erroneous result would cause death or severe disability to the individual, foetus or embryo being tested, or to the individual’s offspring;
(d) for pre-natal screening of women in order to determine their immune status towards transmissible agents;
(e) for determining infective disease status or immune status, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring;
(f) to be used as companion diagnostics;
(g) to be used for disease staging, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring;
(h) to be used in screening, diagnosis, or staging of cancer;
(i) for human genetic testing;
(j) for monitoring of levels of medicinal products, substances or biological components, when there is a risk that an erroneous result will lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring;
(k) for management of patients suffering from a life-threatening disease or condition;
(l) for screening for congenital disorders in the embryo or foetus;
(m) for screening for congenital disorders in new-born babies where failure to detect and treat such disorders could lead to life-threatening situations or severe disabilities.
2.4. Rule 4
(a) Devices intended for self-testing are classified as class C, except for devices for the detection of pregnancy, for fertility testing and for determining cholesterol level, and devices for the detection of glucose, erythrocytes, leucocytes and bacteria in urine, which are classified as class B.
(b) Devices intended for near-patient testing are classified in their own right.
2.5. Rule 5
The following devices are classified as class A:
(a) products for general laboratory use, accessories which possess no critical characteristics, buffer solutions, washing solutions, and general culture media and histological stains, intended by the manufacturer to make them suitable for in vitro diagnostic procedures relating to a specific examination;
(b) instruments intended by the manufacturer specifically to be used for in vitro diagnostic procedures;
(c) specimen receptacles.
2.6. Rule 6
Devices not covered by the above-mentioned classification rules are classified as class B.
2.7. Rule 7
Devices which are controls without a quantitative or qualitative assigned value are classified as class B.
Recent Comments