Per ISO 13485:2016 section 7.3.2, Design and development planning: “The organization shall plan and control the design and development of product.
As appropriate, design and development planning documents shall be maintained and updated as the design and development progresses.
During design and development planning, the organization shall document:
- a) the design and development stages;
- b) the review(s) needed at each design and development stage;
- c) the verification, validation, and design transfer activities that are appropriate at each design and development stage.”
This figure describes the stages, reviews, and the verification, validation, and design transfer activities at each stage.
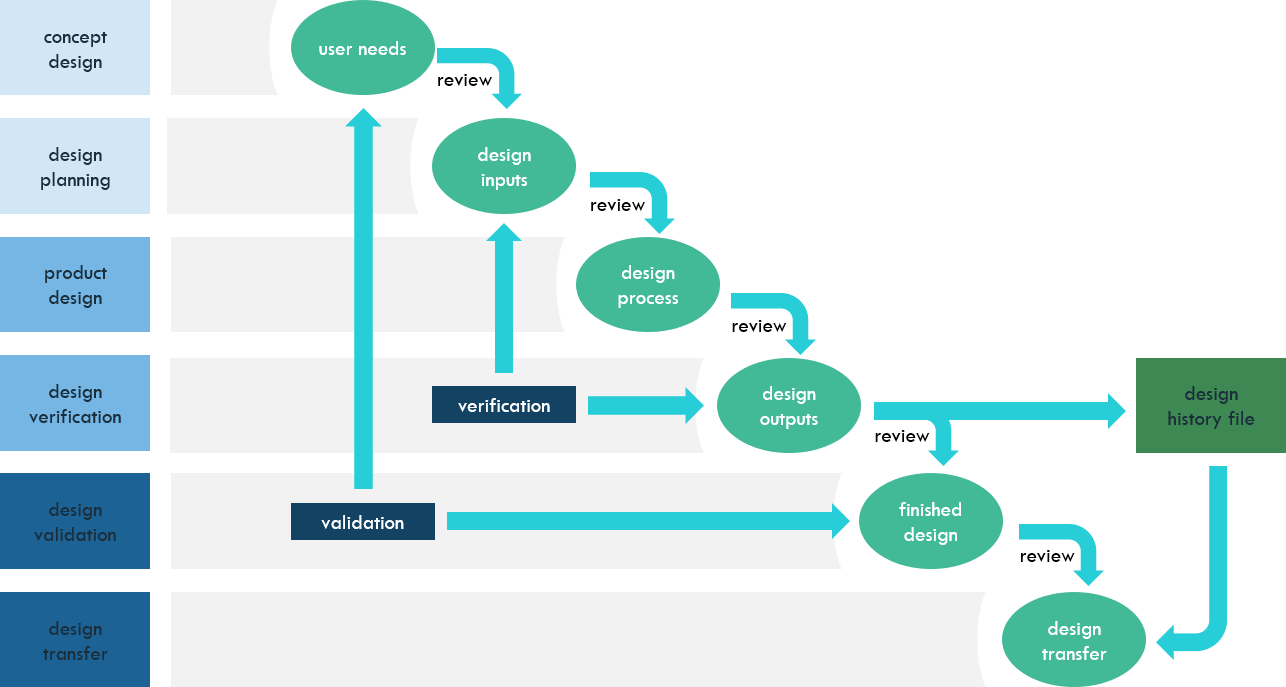
Recent Comments